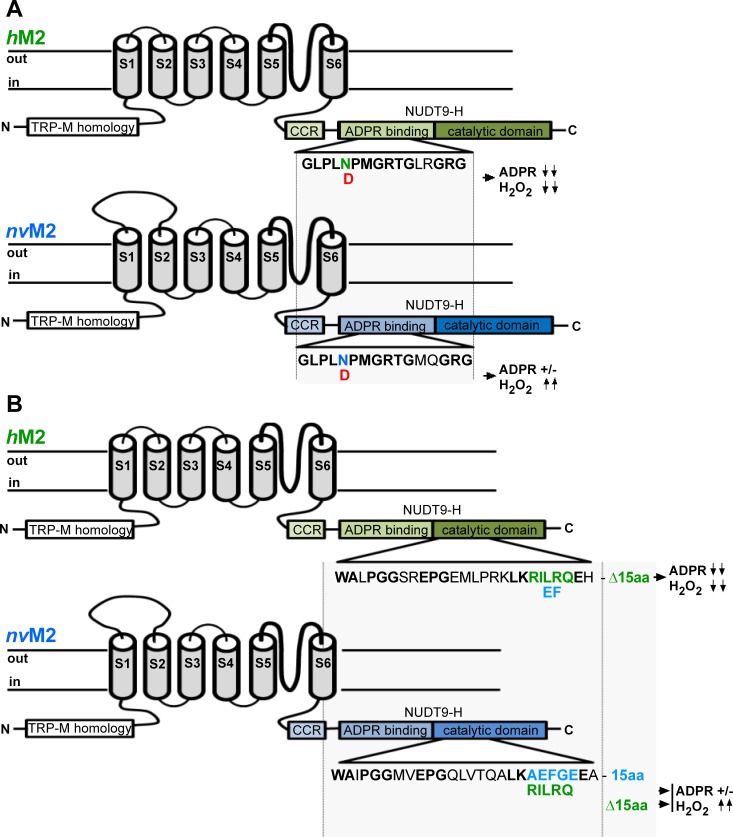
Fig 1. Summary of NUDT9H mutations of hTRPM2 and nvTRPM2 and their effects on channel function.
Characteristic TRPM2 regions like the N-terminal TRPM homology region, the six transmembrane segments, as well as the C-terminal coiled-coiled region and the NUDT9H domain are indicated. The NUDT9H domain is divided into the putative N-terminal ADPR binding region and the C-terminal catalytic domain. (A) Human TRPM2 (green) with the mutation N1326D in the ADPR binding region which abolishes both the sensitivity to ADPR and to H2O2 (symbolized with ↓↓). In contrast, the corresponding mutation N1365D in nvTRPM2 (blue) does not change the sensitivity to ADPR (+/-) but produces strong sensitivity to H2O2 (↑↑). (B) Human TRPM2 in which the double mutation IL to EF within the catalytic site abolishes both the sensitivity to ADPR and to H2O2. The deletion of 15 amino acid residues immediately downstream of the catalytic site which is characteristic for hTRPM2 is also indicated. In contrast, the reciprocal mutations in the catalytic site of nvTRPM2 (EF to IL or the deletion Δ15) again do not change the sensitivity to ADPR but produce sensitivity to H2O2.