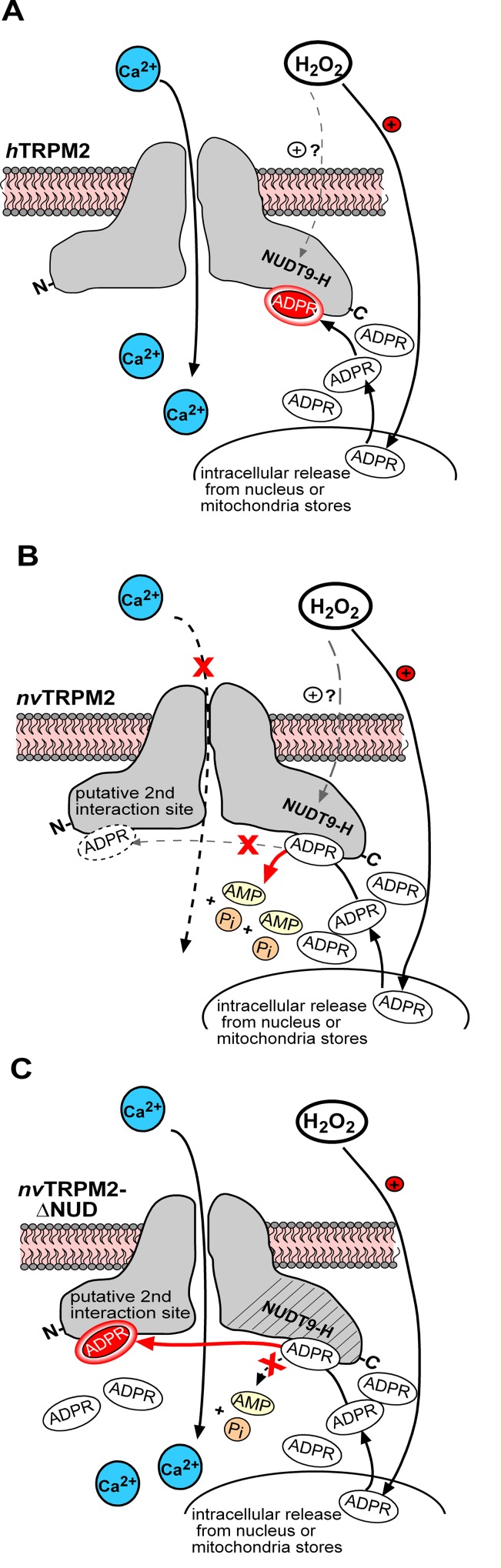
Fig 8. Sketch illustrating the different function of the NUDT9H domain in the two TRPM2 channel orthologs.
(A-C) Cartoon interpretation of the putative functional role of the endogenous NUDT9H domain in heterologeously expressed hTRPM2, nvTRPM2 and nvTRPM2-ΔNUD as indicated during cell exposure to H2O2. It is generally accepted that oxidative stress leads to intracellular release of ADPR. In case of hTRPM2, the accumulated ADPR binds to the NUDT9H domain and initiates channel activation without the requirement of ADPRase activity (A). In contrast, there is a second ADPR interaction site in nvTRPM2 responsible for gating, whereas the NUDT9H domain has a strictly enzymatic role, preventing channel activation by low cytosolic concentrations of ADPR as in the presence of H2O2. Hypothetically, H2O2 may additionally enhance the ADPRase activity of the NUDT9H domain (B). When the catalytic activity of the NUDT9H domain of nvTRPM2 is lost due to point mutations or deletion of the entire domain, increasing concentrations of intracellular ADPR activate the channel (C).