Abstract
Work with sub-natural levels of deuterium (D) in animals has demonstrated an anti-cancer effect of low D-concentration in water. Our objective was to investigate whether deuterium-depleted water (DDW) can overturn reverse manganese (Mn)-induced reduction in life span, using the Caenorhabditis elegans (C. elegans) as a model system. DDW per se had no effect on worm’s life span 48 h after treatment; however, it reversed the Mn-induced decrease in C. elegans life span. Mn reduced DAF-16 levels, a transcription factor strongly associated with life-span regulation. Low D-concentration (90 ppm) restored the Mn-induced changes in DAF-16 to levels indistinguishable from controls, suggesting DDW can regulate the DAF-16 pathway. We further show that insulin-like receptor DAF-2 levels were unaltered by Mn exposure, tAKT levels increased, whilst superoxide dismutase (SOD-3) levels were decreased by Mn. DDW (90 ppm) restored the levels of tAKT and superoxide dismutase (SOD) to control values without changing DAF-2 levels. Treatment of Mn exposed worms with DDW (90 ppm) restored life-span, DAF-16 and SOD-3 levels to control levels, strongly suggesting that low D concentrations can protect against Mn toxic effects.
Keywords: Deuterium depletion, DDW, C. elegans, Life-span, Manganese, DAF-16
1. Introduction
Water is an essential requirement for life. It is the largest constituent of living organisms; the human body consists of 60–70% water dependent upon age. Intra- and extracellular water plays numerous physiological roles, providing an appropriate medium for chemical reactions in cells (Mitchell et al., 1945; Wang et al., 1999).
Hydrogen has a naturally occurring stable isotope, deuterium (D) with a mass number of 2, and it is present in surface water in the form of HDO [semiheavy water; water with light hydrogen (protium, 1H) and deuterium (D or 2H) in the mix] or D2O at a concentration of 16.8 mmol/L or 150 ppm (Katz and Crespi, 1971; Rundel et al., 1988). The two isotopes have the largest mass ratio among stable isotopes of the same element, resulting in significantly different chemical and physical behavior (Jancsó, 2003; Katz and Crespi, 1971; Rundel et al., 1988). Although the effect of D2O – due to its isotopic effect – at an elevated concentration in biological systems has been investigated (Czajka et al., 1961; Katz et al., 1962), the significance of naturally occurring D-concentration has yet to be addressed. It was first reported in 1993 that reduced D concentration in water affects living organisms (Somlyai et al., 1993). In vitro studies noted that D-depletion triggers apoptosis, exerts influence on proto-oncogenes and tumor suppression genes and weakens the expression of genes induced by exposures to carcinogens (Cong et al., 2010; Somlyai et al., 1993, 1998a,b). A study in four patients with brain metastases secondary to lung cancer found that DDW prolonged survival time compared with the average life expectancy in these cases. Survival times of 9.7, 26.6, 33.4, and 54.6 months are unique in the annals of brain metastases secondary to lung tumors (Krempels et al., 2008). In a randomized, double-blind phase 2 study and in a prospective study in patients with prostate cancer consuming DDW parallel to the conventional therapy, prolonged survival was noted (Kovács et al., 2011). Based on these observations, DDW has been posited to offer anticancer activity as an adjunct to conventional therapy.
A link between aging and D is well established. D2O concentrations exceeding the natural level resulted in numerous adverse effects: (a) increased viral mutation rates (Konrad, 1960); (b) deuteration of synthetic estrogen hormones weakened its estrogenic properties (Thompson, 1963); (c) deuterated enzymes exhibited conformational changes, affecting their active sites (Van Hook, 1971); (d) the skin became enriched in deuterium along a temporal aging axis (Griffiths, 1973); (e) reduced the life-span of mice (Czajka and Finkel, 1960). Notably, the effect of lower than natural D concentrations on longevity has yet to be investigated.
Caenorhabditis elegans (C. elegans) offers a unique animal model for aging studies (Baumeister et al., 2006; Lee et al., 2009). This small soil nematode has a short life-cycle, which is significant for assays where the analysis of the whole life-span is necessary (Johnson and Wood, 1982). The progeny is large, and therefore, high throughput assays are possible (Helmcke et al., 2010; Leung et al., 2008). Furthermore, the nematode can be genetically manipulated to provide strains with loss-of-function mutations or complete gene knockout (Brenner, 1974).
DAF-16 is the orthologue of the mammalian FOXO (fork-head O), a transcription factor responsible for activation of genes that codify antioxidant enzymes, which neutralize reactive oxygen species (ROS) involved in the aging process. Notably, daf-16 knockout reduces longevity (Murphy, 2006; Zhang et al., 2009) and, consequently, this pathway has been implicated in the regulation of stress resistance and life-span (Baumeister et al., 2006; Lee et al., 2003). DAF-16 belongs to the DAF-2 insulin-like cascade, which is negatively regulated by phosphorylation. The signaling pathway, via the PI3K receptor DAF2 (insulin-like receptor homologue), regulates levels of 3′-phosphorylated phosphatidylinositol lipids, directing the related kinases AKT1,2 (PKB homologues) to phosphorylate the FOXO homologue, DAF-16 (Hertweck et al., 2004; Murphy, 2006; Paradis and Ruvkun, 1998). This signaling is likely mediated by activation of age1-associated protein (AAP1), the homologue of mammalian heterodimer p85–p110 (Burgering and Kops, 2002). AKT1,2 phosphorylation, in turn, phosphorylates nuclear DAF-16 and translocates it into the cytosol. Conversely, inhibition of this phosphorylation cascade causes the migration of DAF-16 to the nucleus, which binds to DAF-16 binding-element (a core sequence TTGTTTAC of the DNA), thus increasing transcriptional activation of genes with context-dependent effects on cellular stress, consequently reducing oxidative stress and slowing-down the aging process (Murphy, 2006).
Mn has been shown to reduce the life-span in C. elegans (Benedetto et al., 2010) and this model has proven an excellent tool for mechanistically dissecting out aging processes (Baumeister et al., 2006; Hertweck et al., 2004; Lee et al., 2009). Accordingly, the present study was designed to test the hypothesis that DDW has the ability to increase the life span of Mn-exposed worms and that the DAF-16 pathway is modulated by low D concentrations, thus supporting a role for this transcription factor as a key target for pharmacological interventions aimed at prolonging life-span.
2. Materials and methods
2.1. DDW production
DDW was produced from ordinary water containing the natural amount of D (150 ppm, equivalent to16.8 mmol/L), using fractional distillation to decrease the D concentration to 120–90 ppm. The production of DDW is based on the differences between the physical and chemical characteristics of normal water (H2O) and heavy water (D2O). When producing DDW, advantage is taken of the fact that as a consequence of the different volatility, at the boiling point of normal water, the steam in equilibrium with the liquid contains approximately 2.5 percent less deuterium than the liquid phase. By repeating this evaporation (which in industrial quantities is carried out in distillation towers) the deuterium content of water can be decreased, commensurate with the tray number of the distillation tower. To prepare drinking water from the distilled DDW, a stock solution of mineral salts was added or DDW was mixed with mineral water. Mineral salts were supplemented using a stock solution at a final concentration of 3.8 mg/L of KCl, 181.5 mg/L of MgCl2 × 6H2O and 262.5 mg/L of CaCl2 × 2H2O. D concentration was determined by mass spectrometry (Finnigan delta plus XP, using BTW XV standards for the measurement) with ±1 ppm precision.
2.2. C. elegans strains and handling of the worms
C. elegans Bristol N2 (wild type), GR1307 (daf-16 (mgDf50)), MT8313 (ced-3(n2885)), MT7386 (ced-9(n2812); ced-3(n717)) were handled and maintained at 20 °C on Escherichia coli OP50/NGM (nematode growth media) plates as previously described (Brenner, 1974). All strains were provided by the Caenorhabditis Genetics Center (CGC, Minnesota). Synchronous L1 populations were obtained by isolating embryos from gravid hermaphrodites using bleaching solution (1% NaOCl; 0.25 M NaOH), followed by floatation on a sucrose gradient to segregate eggs from dissolved worms and bacterial debris, according to standard procedures (Stiernagle, 1999).
2.3. Treatment with DDW without and with manganese
Synchronized L1 worms were treated with different D-concentrations: 150 ppm D (control); 120 ppm D and 90 ppm D for 48 h. The treatment was performed in a liquid media and in the presence of oxygen and food. After the treatment, worms were washed with 85 mM NaCl in order to remove the DDW and then transferred to new NGM/OP50 to allow recovery and growth through the L4 stage. To test the efficacy of DDW in attenuating experimentally induced shortened life-span, we used an established model (Baumeister et al., 2006; Benedetto et al., 2010). Briefly, L1 worms were exposed to Mn (35 mM) for 30 min prior to DDW treatment. After washing off the Mn with 85 mM NaCl for three times, worms were then treated for additional 48 h with control (regular distilled tap water containing 150 ppm D) or DDW (120 and 90 ppm). After this period, DDW was washed off with 85 mM NaCl and worms were placed on plates for life-span experiments or prepared for homogenization for western blot analysis, as described below.
2.4. Life-span experiments
Aged L4 and healthy-looking worms previously treated with DDW without or with exposure to Mn (around 30 condition in duplicates) were transferred to new OP50-seeded NGM plates containing FUDR (5-fluorodeoxyuridine). Worms were transferred to new plates every 3 days for feeding until 60% of the animals died. Survival was assessed daily until all the worms died. All tested C. elegans strains were assessed in parallel, and each experiment was performed in triplicates. Plotted curves represent averages of triplicate independent experiments.
2.5. Western blot assay
Twenty thousand worms treated as previously described were washed off to remove DDW and then homogenized by sonication in a lysis buffer [85 mM NaCl, 1% Triton X-100, 10 mM Tris Buffer (pH 6.8), 1× protease inhibitor and 50 mM dithiotreitol (DTT)]. After centrifugation (11,000 × g for 1 min), the supernatant was isolated and the protein concentration was determined with the Bradford method (Bradford, 1976). One hundred micrograms of proteins were treated with sample buffer (Laemni sample buffer – Bio Rad) and after 5-min boiling were applied to an acrylamide-based gel for electrophoresis. The proteins were then transferred to a nitrocellulose membrane, which was incubated with primary antibodies for the protein of interest [DAF-2 (1:5000, sc9232), DAF-16 (1: 5000, sc33738), tAKT (1: 5000, sc-1619), iron/manganese SOD-3 (1: 5000, ab13533)] and visualized by horseradish peroxidase conjugated secondary antibody. Purified β-actin (1:10,000 – A1978, Sigma, St. Louis, MO) was used as a control and the density of the bands obtained after development was acquired with Image J (National Institutes of Health, Bethesda, MD, USA, http://imagej.nih.gov/ij/)
2.6. Statistical analysis
Life-span curves and western blot analyses were generated with GraphPad Prism (GraphPad Software Inc.). We used a sigmoidal dose-response model with a top constraint at 100% to draw the curves and determine the life-span values reported in the graphs. Statistical analysis of significance was carried out by repeated measures MANOVA or one-way ANOVA followed by post hoc Bonferroni test when the overall p value was less than 0.05. The error bars represent SEMs.
3. Results
3.1. DDW effects on life span
Fig. 1 shows that 48-h treatment with DDW alone did not affect life-span in C. elegans at either of the two tested concentrations.
Fig. 1.
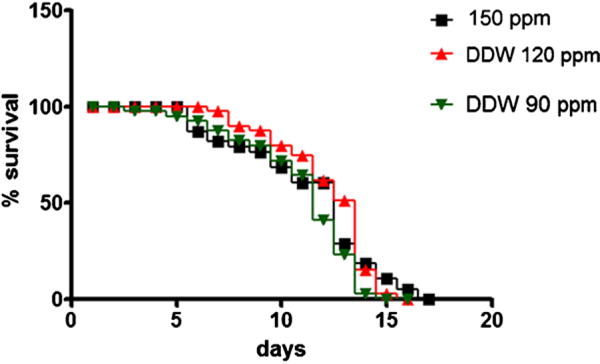
Life-span of wild-type C. elegans treated with different D-concentrations. Closed squares are controls (150 ppm); upwardly pointing triangles were treated with DDW 120 ppm; downwardly pointing triangles were treated with DDW 90 ppm. Data are expressed as mean of 3 different experiments.
3.2. DDW protects from Mn-induced aging
When worms were pre-exposed to Mn, there was a statistically significant reduction in their life-span (Fig. 2, p < 0.05). In parallel experiments, at the end of DDW treatment the proteins were extracted for further analysis. Mn exposure caused a decrease in DAF-16 levels, which was also reflected in decreased levels of a downstream gene product, SOD (Fig. 3A and B). Upstream to DAF-16, AKT was increased, indicating Mn-induced aberrant signaling in DAF-16 (Fig. 3C). However, expression levels of the upstream effector of the cascade, the membrane receptor DAF-2 were not altered by Mn (data not shown).
Fig. 2.
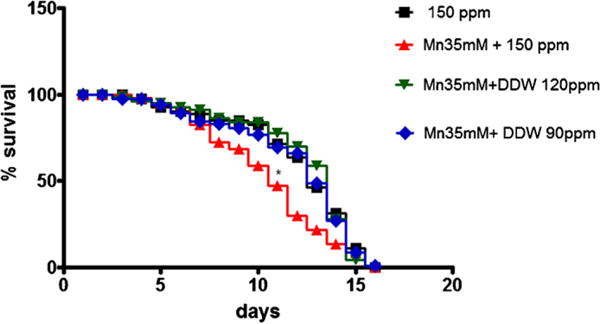
Life-span of wild-type C. elegans pre-exposed to Mn (35 mM) for 30 min and then treated with DDW for 48 h. Squares represent control group (150 ppm); upwardly pointing triangles represent worms exposed to Mn 35 mM; downwardly pointing triangles represent Mn 35 mM + DDW 120 ppm and diamonds represent Mn 35 mM + DDW 90 ppm. Data are expressed as mean of 3 independent experiments. Error bars were omitted for better visualization of the graphics. * indicates statistical difference from control 150 ppm group at p < 0.05.
Fig. 3.
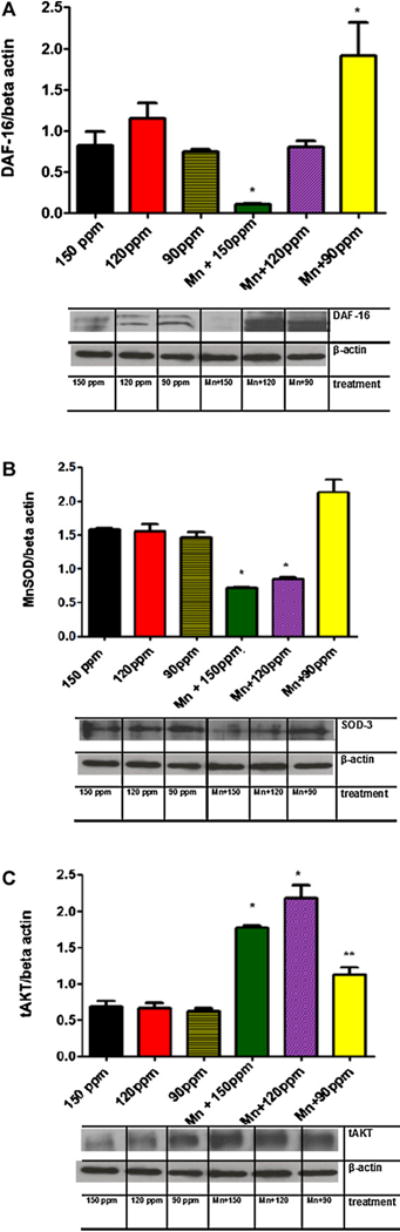
Protein levels detected by Western blot assay in wild-type C. elegans. (A) DAF-16 levels; (B) SOD levels; (C) tAKT levels. Bars represent mean ± SEM. * indicate statistical difference from control (150 ppm) group (p < 0.05) and ** indicates difference from the Mn 35 mM group (p < 0.05). Data are expressed as mean of three different experiments ± SEM.
Treatment with DDW at both 120 and 90 ppm D concentrations caused significant protection from reduced life-span induced by Mn (Fig. 2, p < 0.05). DDW (120 ppm) reversed the Mn-induced reduction in DAF-16 protein levels (p < 0.05; Fig. 3A) to levels indistinguishable from controls. Notably, DAF-16 levels in the group Mn + DDW 90 ppm were significantly higher than control levels. Furthermore, SOD and AKT protein levels were restored to control levels at the lower D concentration (90 ppm; Fig. 3B and C, respectively, p < 0.05).
To investigate whether DAF-16 is exclusively involved in the ability of DDW to mitigate the Mn-induced reduction in life-span, the daf-16 (mgDf50) loss-of-function mutant was examined. The mutants showed significantly shortened life-span compared to wild-type when exposed to Mn (Fig. 4, p < 0.05), indicating that daf-16 may not be the only gene associated to Mn-induced reduction in life span. Furthermore, DDW restored this aging effect of Mn at both DDW concentrations (120 and 90 ppm) (Fig. 4, p < 0.05).
Fig. 4.
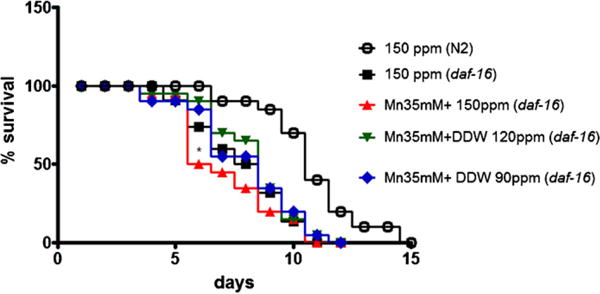
Effects of DDW vs. Mn on the life-span of daf-16 (mgDf50). Open circles represent control N2 worms (150 ppm); Squares represent daf-16 control group (150 ppm); upwardly pointing triangles represent worms exposed to Mn 35 mM; downwardly pointing triangles represent Mn 35 mM + DDW 120 ppm and diamonds represent Mn 35 mM + DDW 90 ppm. Data are expressed as mean of 3 independent experiments. Error bars were omitted for better visualization of the graphics. * indicates statistical difference from control (150 ppm) group at p < 0.05.
3.3. Apoptosis may be involved in DDW’s effect
Previous studies showed the ability of DDW to induce apoptosis in several tumor cell lines (Somlyai et al., 2010). Accordingly, we investigated whether the apoptotic pathway is associated with the increased life-span provided by DDW after Mn exposure. Ced-3 knockdown caused a not significant increased in the life-span of untreated worms and Mn effect on reducing life-span was no longer detected (Fig. 5A). Similarly, the double-KO worms ced-3, ced-9 were not affected by Mn exposure (Fig. 5B). Treatment with DDW following Mn exposure did not affect the life-span either in ced-3 or ced-3; ced-9 double mutants.
Fig. 5.
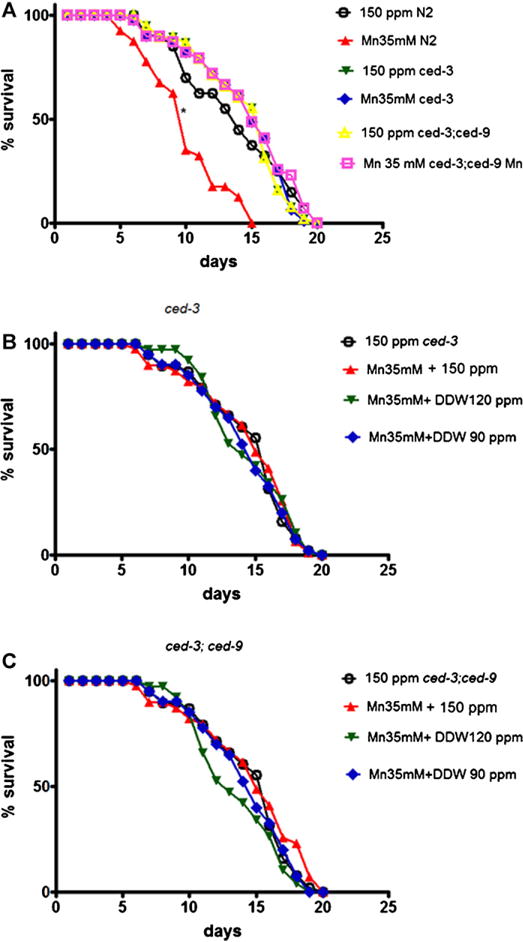
Life-span of ced-3 and ced-3;ced-9 mutants followed Mn vs. DDW treatments. (A) Comparison between N2, ced-3 and ced-3;ced-9 with and without Mn treatment. Open circles represent N2 control (150 ppm) group; closed upwardly pointing triangles represents N2 exposed to Mn; closed downwardly pointing triangles represents ced-3 control (150 ppm) group; diamonds represents ced-3 exposed to Mn; open triangle represents ced-3;ced-9 control (150 ppm) group and open square represents ced-3;ced-9 Mn exposed group; (B) ced-3 life-span-open circle represents control group (150 ppm); upwardly pointing triangles represent worms exposed to Mn 35 mM; downwardly pointing triangles represent Mn 35 mM + DDW 120 ppm and diamonds represent Mn 35 mM + DDW 90 ppm; (C) ced-3; ced-9 life-span-open circle represents control group (150 ppm); upwardly pointing triangles represent worms exposed to Mn 35 mM; downwardly pointing triangles represent Mn 35 mM + DDW 120 ppm and diamonds represent Mn 35 mM + DDW 90 ppm. Data are expressed as mean of 3 independent experiments. Error bars were omitted for better visualization of the graphic. * indicates statistical difference from control (150 ppm) group (p < 0.05).
4. Discussion
Though the concentration of D in all living organisms is greater than 10 mmol/L, its biological role has yet to be defined. Recent studies have shown that depletion of naturally occurring D can result in tumor regression in mice, dogs, cats and humans (Kovács et al., 2011; Krempels et al., 2008; Somlyai et al., 1998a,b). The present study demonstrates that subnormal D concentrations reversed the Mn-induced reduction in the nematode’s life-span and modified the DAF-16 signaling cascade. Accordingly, changes in the D/H ratio can trigger molecular processes that regulate gene expression participating in the DAF-16/FOXO cascade.
Our study showed that DDW itself failed to increase life-span per se. The effect on the longevity was only detected when worms were exposed to chemical stress, namely 35 mM Mn. A study by Zhang et al., also reported that the main active ingredient of green tea, epigallocathechin gallate, extended significantly C. elegans life-span, but only under conditions of stress, analogous to the findings herein (Zhang et al., 2009). It is noteworthy that our treatment protocol used 48-h exposure, which is relatively long within the context of the worm’s life-span.
Along with reduced longevity, Mn exposure also reduced levels of DAF-16 in the worms (Fig. 3A). Decreased DAF-16 levels or daf-16 gene deletion have been shown to cause ~25% reduction in the worm’s life-span (Baumeister et al., 2006; Lee et al., 2003; Zhang et al., 2009). It was hypothesized that this reduction was caused by increased susceptibility to oxidative stress. As DAF-16 migration from the cytosol to the mitochondria is essential for the activation and expression of antioxidant enzymes, such as SOD, the absence of this transcription factor likely generates an imbalance in the pro-oxidant/antioxidant homeostasis, causing increased ROS production, mitochondrial dysfunction, cell death and, consequently, shorter life-span. Reinforcing this hypothesis, pro-oxidant agents such as paraquat and cadmium reduce life-span as well as DAF-16 protein levels (Guan et al., 2010; Tvermoes et al., 2010). In order to determine which step in the DAF-16 cascade was negatively modulated by Mn, we analyzed the levels of the insulin-like receptor DAF-2; the levels of the intermediary kinase AKT; and also a DAF-16 target, SOD-3, a mitochondrial iron/manganese-dependent antioxidant enzyme. Mn did not modify DAF-2 protein levels, however it increased AKT levels. Consistent with these findings, Mn also reduced the levels of the enzymatic antioxidant SOD-3. This reduction was fully restored by DDW 90 ppm, indicating that increased levels of DAF-16 caused at this D concentration (Fig. 3A) are essential for the recovery of the enzyme. This effect, along with the increased life-span, indicates that DDW exerts action by modulating the DAF-16 cascade. Furthermore, this modulation occurs at a point downstream to the DAF-2 receptor, attesting to an intracellular effect. However, since DDW also increased the life-span in the absence of DAF-16 in the daf-16 (mgDf50) mutant, the reduced D concentration may also affect other signaling pathways in restoring the pro-oxidant/antioxidant balance.
Earlier studies have shown that DDW induced apoptosis of cancer cells both in vitro and in vivo (Cong et al., 2010; Somlyai et al., 1993, 1998b). Our study has shown that the absence of CED-3, a caspase directly involved in apoptosis, may be also involved on Mn-induced toxicity. This can be suggested because the absence of this caspase caused a loss of the Mn toxic effect. In fact, apoptosis has been associated to Mn toxicity in cultured neuronal cells (Deng et al., 2011; Yoon et al., 2011). Similarly, the double mutant ced-3;ced-9 also has shown the same effect as the single mutant ced-3. Accordingly to Shaham and Horvitz (1996), ced-9 is a protective gene and its loss-of function lead cells that normally live undergo to apoptosis (Shaham and Horvitz, 1996). We believe that because of the double mutation, we could not observe the effects of ced-9 mutation, implicating that ced-4, another important caspase inducer gene, which has been shown to be regulated by ced-9, may not be associated to Mn-induced apoptosis. Therefore, our data on apoptosis are still very little and further evaluations must be performed in order to assure the apoptosis role on Mn-induced toxicity in C. elegans.
Taken together, the present study shows that DDW reversed the shortened life span induced by Mn exposure, restoring DAF-16 and SOD-3 levels in C. elegans. The results do not support the hypothesis that DDW per se increases the life-span in the worms, most likely due to the short exposure paradigm. In fact, we cannot rule out that long term application of DDW will increase the life-span by reducing the number of gene alterations. Considering that the main pharmaceutical intervention with DDW is in cancer therapy (where conventional treatments cause strong side effects), future studies should further evaluate whether the application of DDW may be used to prevent or reduce the severity of the cytotoxic effect associated with conventional chemotherapies.
5. Conclusions
The present investigation shows that deuterium depletion in water reverses the intracellular effects of Mn exposure in C. elegans. We show that Mn caused reduction in DAF-16 and SOD-3 levels, which was associated with reduced life-span. Notably, treatment of Mn exposed worms with DDW (90 ppm) restored life-span, DAF-16 and SOD-3 levels to control levels. In conclusion, our study strongly suggest that low D concentrations can restore the Mn-induced reduced life-span in C. elegans, reiforcing the need of further studies to improve the understanding on DDW therapeutic mechanisms.
Acknowledgments
NIH ES R01 10563 and 07331 (MA).
Footnotes
Conflict of interest
There is no conflict of interest in this study and in the preparation of this manuscript.
References
- Baumeister R, Schaffitzel E, Hertweck M. Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. Journal of Endocrinology. 2006;190:191–202. doi: 10.1677/joe.1.06856. [DOI] [PubMed] [Google Scholar]
- Benedetto A, Au C, Avila DS, Milatovic D, Aschner M. Extracellular dopamine potentiates mn-induced oxidative stress, life-span reduction, and dopaminergic neurodegeneration in a BLI-3-dependent manner in Caenorhabditis elegans. PLoS Genetics. 2010;6 doi: 10.1371/journal.pgen.1001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends in Biochemical Sciences. 2002;27:352–360. doi: 10.1016/s0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- Cong F, Zhang Y, Sheng H, Ao Z, Zhang S, Wang J. Deuterium-depleted water inhibits human lung carcinoma cell growth by apoptosis. Experimental Therapeutical Medicine. 2010;1:277–283. doi: 10.3892/etm_00000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajka DM, Finkel AJ. Effect of deuterium oxide on the reproductive potential of mice. Annals of the New York Academy of Sciences. 1960;84:770–779. doi: 10.1111/j.1749-6632.1960.tb39109.x. [DOI] [PubMed] [Google Scholar]
- Czajka DM, Finkel AJ, Fischer CS, Katz JJ. Physiological effects of deuterium on dogs. American Journal of Physiology. 1961;201:357–362. doi: 10.1152/ajplegacy.1961.201.2.357. [DOI] [PubMed] [Google Scholar]
- Deng Y, Xu D, Xu B, Xu Z, Tian Y, Feng W, Liu W, Yang H. G0/G1 phase arrest and apoptosis induced by manganese chloride on cultured rat astrocytes and protective effects of riluzole. Biological Trace Element Research. 2011;144:832–842. doi: 10.1007/s12011-011-9028-7. [DOI] [PubMed] [Google Scholar]
- Griffiths TR. A new unifying theory for the initiation of ageing mechanisms and processes. Mechanisms of Ageing and Development. 1973;2:295–307. doi: 10.1016/0047-6374(73)90024-9. [DOI] [PubMed] [Google Scholar]
- Guan S, Li P, Luo J, Li Y, Huang L, Wang G, Zhu L, Fan H, Li W, Wang L. A deuterohemin peptide extends life-span and increases stress resistance in Caenorhabditis elegans. Free Radical Research. 2010;44:813–820. doi: 10.3109/10715762.2010.485991. [DOI] [PubMed] [Google Scholar]
- Helmcke KJ, Avila DS, Aschner M. Utility of Caenorhabditis elegans in high throughput neurotoxicological research. Neurotoxicology and Teratology. 2010;32:62–67. doi: 10.1016/j.ntt.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Hertweck M, Gobel C, Baumeister R. C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Developmental Cell. 2004;6:577–588. doi: 10.1016/s1534-5807(04)00095-4. [DOI] [PubMed] [Google Scholar]
- Jancsó G. Isotope effects. In: Vértes A, Nagy S, Klencsár Z, editors. Handbook of Nuclear Chemistry. Vol. 2. Kluwer Academic Publishers; Dordrecht, Netherlands: 2003. pp. 85–116. [Google Scholar]
- Johnson TE, Wood WB. Genetic analysis of life-span in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:6603–6607. doi: 10.1073/pnas.79.21.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JJ, Crespi HL. Isotope effects in biological systems. In: Collins CJ, Bowman NS, editors. Isotope Effects in Chemical Reactions. Van Nostrand Reinhold; New York, NY, USA: 1971. pp. 286–363. [Google Scholar]
- Katz JJ, Crespi HL, Czajka DM, Finkel AJ. Course of deuteriation and some physiological effects of deuterium in mice. American Journal of Physiology. 1962;203:907–913. doi: 10.1152/ajplegacy.1962.203.5.907. [DOI] [PubMed] [Google Scholar]
- Konrad M. The mutagenic effect of D20 on bacteriophage T4. Annals of the New York Academy of Sciences. 1960;84:678–684. doi: 10.1111/j.1749-6632.1960.tb39100.x. [DOI] [PubMed] [Google Scholar]
- Kovács A, Guller I, Krempels K, Somlyai I, Jánosi I, Gyomgyi Z, Szabó I, Ember IGS. Deuterium depletion may delay the progression of prostate cancer. Journal of Cancer Therapy. 2011;2:548–556. http://dx.doi.org/10.4236/jct.2011.24075. [Google Scholar]
- Krempels K, Somlyai I, Somlyai G. A retrospective evaluation of the effects of deuterium depleted water consumption on 4 patients with brain metastases from lung cancer. Integrative Cancer Therapies. 2008;7:172–181. doi: 10.1177/1534735408322851. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Murphy CT, Kenyon C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metabolism. 2009;10:379–391. doi: 10.1016/j.cmet.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Kennedy S, Tolonen AC, Ruvkun G. DAF-16 target genes that control C. elegans life-span and metabolism. Science. 2003;300:644–647. doi: 10.1126/science.1083614. [DOI] [PubMed] [Google Scholar]
- Leung MC, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, Meyer JN. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicological Sciences. 2008;106:5–28. doi: 10.1093/toxsci/kfn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell HH, Hamilton TS, Steggerda FR, Bean HW. The chemical composition of the adult human body and its bearing on the biochemistry of growth. Journal of Biological Chemistry. 1945;158:625–637. [Google Scholar]
- Murphy CT. The search for DAF-16/FOXO transcriptional targets: approaches and discoveries. Experimental Gerontology. 2006;41:910–921. doi: 10.1016/j.exger.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes and Development. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundel PW, Ehleringer JR, Nagy KA. Stable Isotopes in Ecological Research. Springer; New York, NY, USA: 1988. [Google Scholar]
- Shaham S, Horvitz HR. Developing Caenorhabditis elegans neurons may contain both cell-death protective and killer activities. Genes and Development. 1996;10:578–591. doi: 10.1101/gad.10.5.578. [DOI] [PubMed] [Google Scholar]
- Somlyai G, Laskay G, Berkényi T, Galbács Z, Kiss GA, Gy J. The biological effects of deuterium-depleted water, a possible new tool in cancer therapy. Journal of Oncology. 1998a;30:91–94. [Google Scholar]
- Somlyai G, Laskay G, Berkényi T, Jákli Gy, Jancsó G. Naturally occurring deuterium may have a central role in cell signalling. In: Heys JR, Melillo D, editors. Synthesis and Applications of Isotopically Labelled Compounds. John Wiley and Sons Ltd; New York: 1998b. pp. 137–141. [Google Scholar]
- Somlyai G, Jancso G, Jakli G, Vass K, Barna B, Lakics V, Gaal T. Naturally occurring deuterium is essential for the normal growth rate of cells. FEBS Letters. 1993;317:1–4. doi: 10.1016/0014-5793(93)81479-j. [DOI] [PubMed] [Google Scholar]
- Somlyai G, Molnar M, Laskay G, Szabo M, Berkenyi T, Guller I, Kovacs A. Biological significance of naturally occurring deuterium: the antitumor effect of deuterium depletion. Orvosi Hetilap. 2010;151:1455–1460. doi: 10.1556/OH.2010.28865. [DOI] [PubMed] [Google Scholar]
- Stiernagle T. Maintenance of C. elegans. In: Hope IA, editor. C elegans: A Practical Approach. Oxford University Press; New York: 1999. [Google Scholar]
- Thompson J. Biological Effects of Deuterium. Pergamon Press, Modern Trends in Physiological Sciences Division; 1963. (International Series of Monographs on Pure and Applied Biology). [Google Scholar]
- Tvermoes BE, Boyd WA, Freedman JH. Molecular characterization of numr-1 and numr-2: genes that increase both resistance to metal-induced stress and life-span in Caenorhabditis elegans. Journal of Cell Science. 2010;123:2124–2134. doi: 10.1242/jcs.065433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hook W. Kinetic isotope effects: introduction and discussion of the theory. In: Bowman CJCaNS., editor. Isotope Effects in Chemical Reactions. Van Nostrand Reinhold; New York: 1971. pp. 1–89. [Google Scholar]
- Wang Z, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, Heymsfield SB. Hydration of fat-free body mass: review and critique of a classic body-composition constant. American Journal of Clinical Nutrition. 1999;69:833–841. doi: 10.1093/ajcn/69.5.833. [DOI] [PubMed] [Google Scholar]
- Yoon H, Kim DS, Lee GH, Kim KW, Kim HR, Chae HJ. Apoptosis induced by manganese on neuronal SK-N-MC cell line: endoplasmic reticulum (ER) stress and mitochondria dysfunction. Environmental Health and Toxicology. 2011;26:e2011017. doi: 10.5620/eht.2011.26.e2011017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Jie G, Zhang J, Zhao B. Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Radical Biology and Medicine. 2009;46:414–421. doi: 10.1016/j.freeradbiomed.2008.10.041. [DOI] [PubMed] [Google Scholar]


