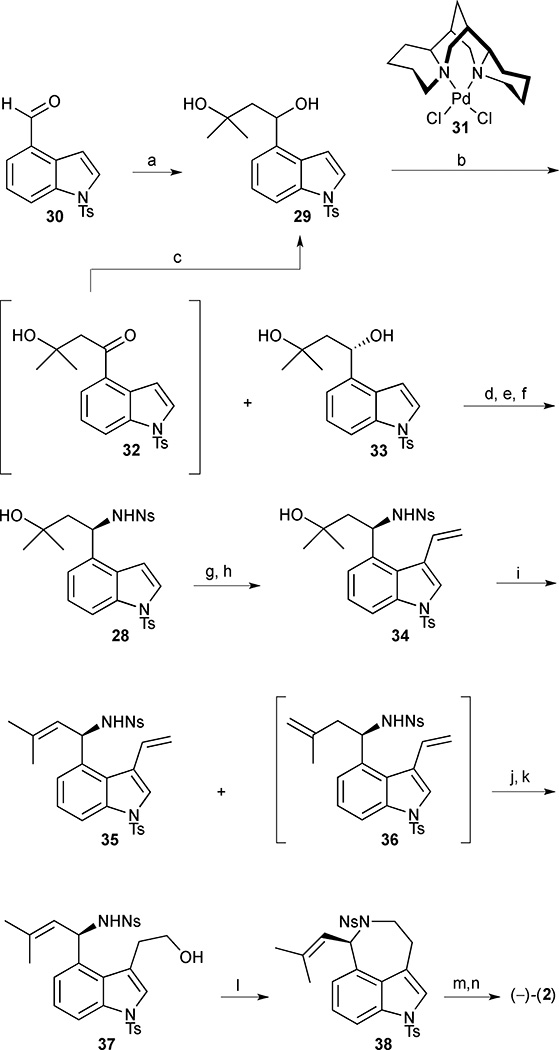
Scheme 1.
(−)-Aurantioclavine (2). Reagents and conditions: (a) isobutylene oxide, lithium 4,4'-ditert-butylbiphenylide (LiDBB), THF, −78 °C, 69%; (b) [Pd((−)-sparteine)Cl2] (31) (10 mol%), O2 (1 atm), (−)-sparteine (40 mol%), 3Å MS, t-BuOH, 40 °C to 70 °C, 98 h, 32, 51%, and 33, 37% (96% ee); (c) LiAlH4, THF, −78 °C, 95%; (d) HN3, PBu3, DIAD, PhMe, −78 °C to −20 °C, 80%; (e) H2, cat. Pd/C, HCl, MeOH, 23 °C; (f) o-NsCl, Et3N, CH2Cl2, 0 °C to 23 °C, 92% (2 steps); (g) PyHBr3, CH2Cl2, 0 °C to 23 °C, 72%; (h) tributylvinyltin, Pd(PPh3)4 (20 mol%), PhMe, 100 °C, 75%; (i) POCl3, pyridine, 0 °C to 23 °C, 95%, 35:36 (68:32); (j) 9-BBN, THF, 23 °C (k) NaOH, H2O2, THF/EtOH/H2O, 0 °C to 23 °C, 48% (2 steps); (l) DIAD, PPh3, PhMe, 0 °C, 95%; (m) PhSH, K2CO3, DMF, 23 °C, 53%; (n) TBAF, THF, 70 °C, 68%.