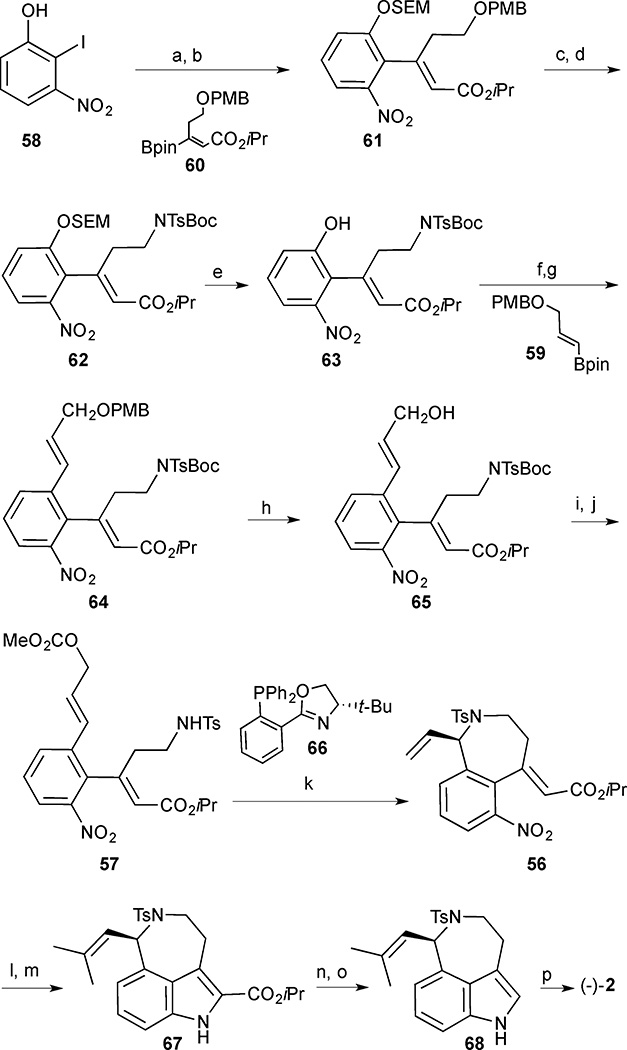
Scheme 4.
(−)-Aurantioclavine (2). Reagents and conditions: (a) SEMCl, Cs2CO3, CH3CN, 96%; (b) Pd(OAc)2, SPhos, Na2CO3, DMF, 100 °C, vinylborane 60; (c) DDQ, CH2Cl2/H2O, 82% (2 steps); (d) BocNHTs, DIAD, PPh3, THF, 88%; (e) conc. HCl, MeOH, CH2Cl2, 70%; (f) PhNTf2, Et3N, CH2Cl2, 87%; (g) vinylborane 59, Pd(PPh3)4, Na2CO3, EtOH/PhMe/H2O, 100 °C; (h) DDQ, CH2Cl2/H2O, 84% (2 steps); (i) ClCO2Me, pyridine, CHCl3, reflux; (j) TFA, CHCl3, reflux, 87% (2 steps); (k) tBu-PHOX 66 (45 mol%), Pd2(dba)3 (15 mol%), Bu4NCl (0.30 equiv.), 0 °C, CH2Cl2, 72 h, 77% (95% ee); (l) 2-methyl-2-butene, Grubbs 2nd generation catalyst., 40 °C; (m) P(OEt)3, 170 °C, 78% (2 steps); (n) NaOH (aq), THF/MeOH, reflux; (o) Cu, quinoline, 190 °C, 68% (2 steps); (p) Na/ naphthalene, DME, −78 °C, 90%