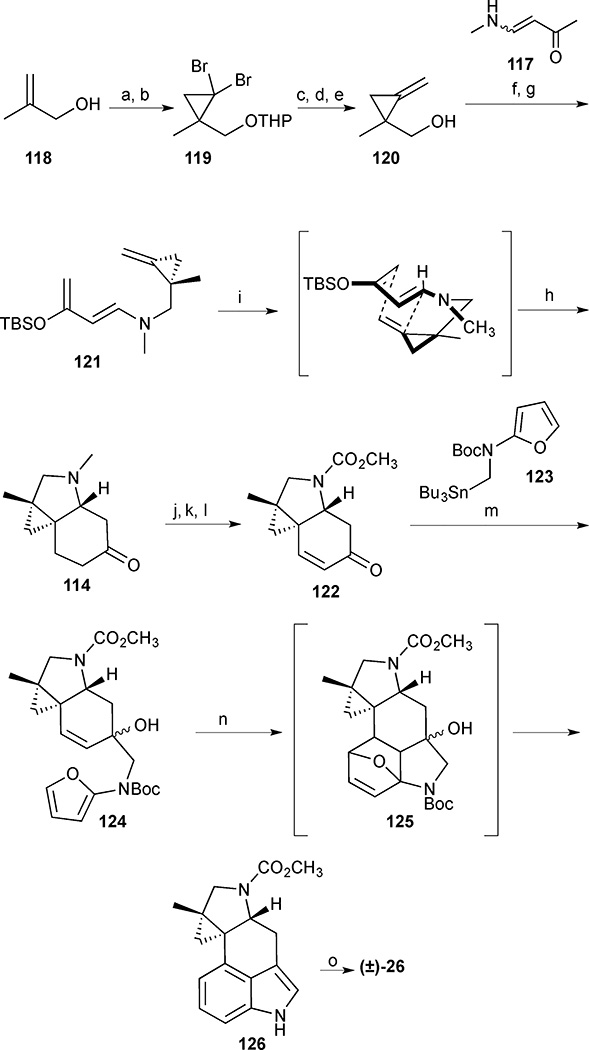
Scheme 13.
Cycloclavine (26). Reagents and conditions: (a) DHP, HCl (cat.), 90%; (b) CHBr3, Et3N, cetrimide, NaOH (aq), CH2Cl2, 95%; (c) n-BuLi, THF, −95 °C then MeI, −95 °C to room temperature, 82%; (d) KOt-Bu, DMSO, room temperature, 69%; (e) p-TsOH, MeOH, room temperature, 79%; (f) MsCl, Et3N, CH2Cl2, 0 °C, 1 h; (g) amide 117, NaH, DMF, room temperature, 12 h, 67% (2 steps); (h) NaHMDS, THF, −78 °C then TBSCl; (i) 195 °C, PhCF3, µw, 1 h 52%, (72% brsm) (2 steps); (j) TBAF, THF, room temperature, 85%; (k) MeOC(O)Cl, 70 °C, 3 h, 71%; (l) LDA, THF, −78 °C, 1 h then TMSCl (1.3 equiv.), CH3CN, 12 h, 67%; (m) 123, n-BuLi, THF, −78 °C, 51%; (n) 180 °C, PhCF3, µW, 30 min, 44% (56% brsm); (o) LiAlH4, THF, 66 °C, 30 min, quantitative.