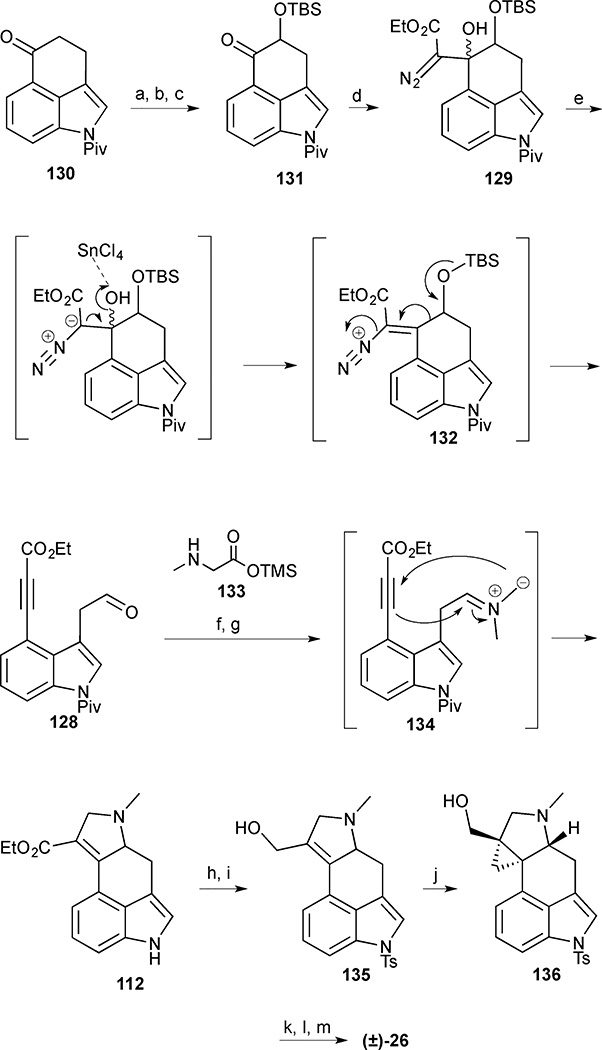
Scheme 14.
Cycloclavine (26). Reagents and conditions: (a) TBSOTf, Et3N, CH2Cl2, 0 °C to room temperature, 45 min; (b) mCPBA, K2CO3, CH2Cl2, 0 °C, 2 h; (c) TBSCl, DMAP, imidazole, CH2Cl2, 6 h, 70% (3 steps); (d) ethyl diazoacetate, LDA, THF, −78 °C, 2 h, 79%; (e) SnCl4, CH2Cl2, 0 °C, 10 min, 80%; (f) trimethylsilyl methylglycinate (133), PhMe, 0 °C, 30 min then 120 °C, 30 min, 65%; (g) DBU, H2O, THF, reflux, 19 h; (h) TsCl, Bu4NHSO4, KOH, PhMe, 78% (2 steps); (i) DIBAL-H, PhMe, 0 °C to room temperature, 30 min; (j) Et2Zn, I2, CH3I, CH2Cl2, 0 °C to room temperature, 24% (2 steps); (k) MsCl, NEt3, 0 °C, 1 h; (l) LiBHEt3, THF, 0 °C, 45 min; (m) NaOH, MeOH, reflux, 21% (3 steps)