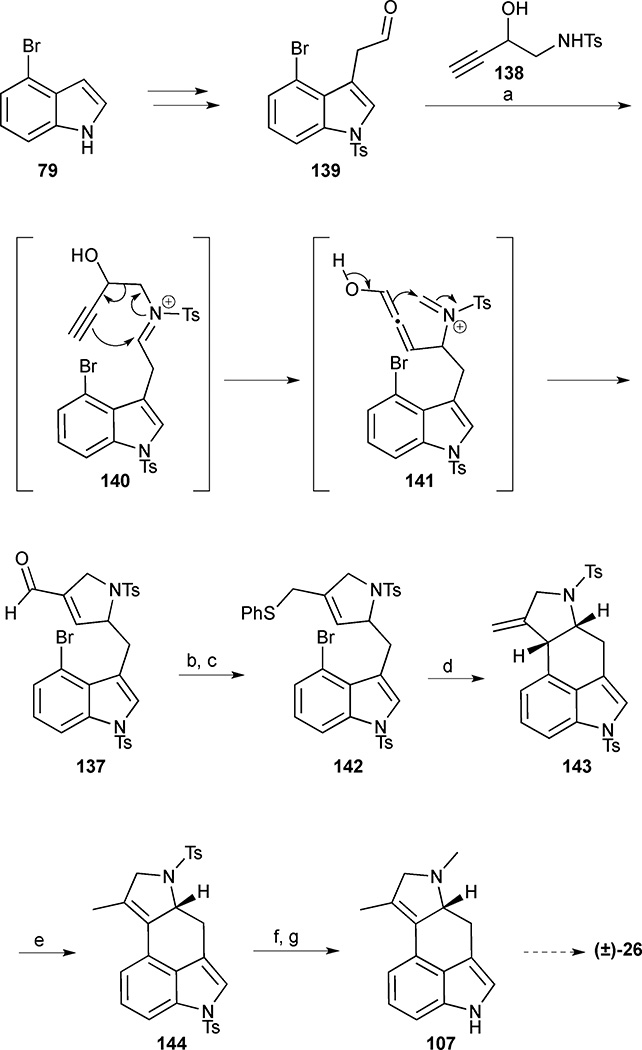
Scheme 15.
Cycloclavine (26). Reagents and conditions: (a) 2-hydroxy homopropargyl tosylamine (138), FeCl3, CH2Cl2, reflux, 0.2 h, 83%; (b) NaBH4, CeCl3•7H2O, MeOH, 0 °C; (c) PhSSPh, n-Bu3P, benzene, 81% (2 steps); (d) n-Bu3SnH, AIBN, benzene, reflux, 91%; (e) p-TsOH•H2O, benzene, reflux, 63%; (f) sodium naphthalenide, THF, −78 °C; (g) formalin, AcOH, NaBH3CN, THF, 71% (2 steps).