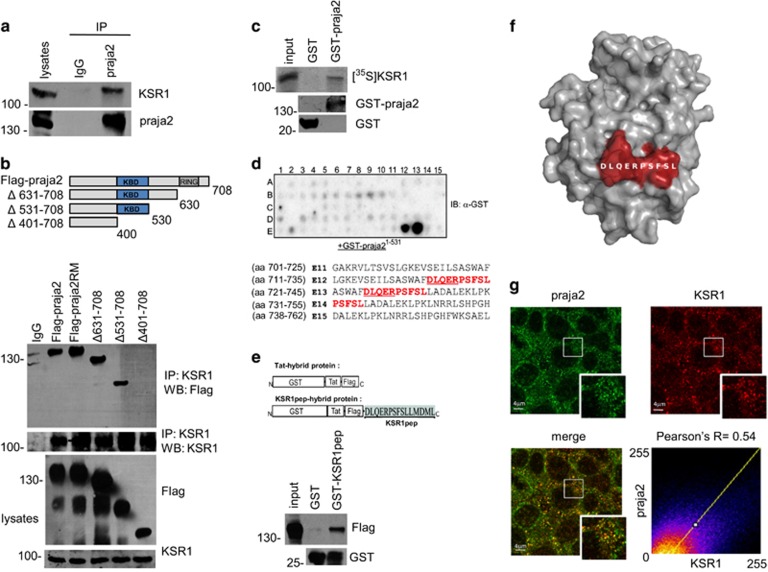
Figure 1.
praja2 interacts with KSR1. (a) Isolation of endogenous KSR1 and praja2 complex from lysates (2 mg) of HEK293 cells. (b) Schematic representation of the praja2 constructs used (upper diagram). HEK293 cells were transiently transfected with flag-praja2 (either wild-type, ring mutant (RM) or deletion mutants). Cells were treated for 12 h with MG132 (10 μM) before harvesting. Twenty-four hours following transfection, cells were harvested and lysed. Lysates were subjected to immunoprecipitation with anti-KSR1 antibody. Precipitates were immunoblotted with anti-KSR1 and anti-flag antibodies (lower panels). (c) In vitro translated, [35S]-labeled KSR1 was subjected to pull-down assays with purified GST or GST–praja2 fusion. (d) Spotted peptides (25 mers, 15mer overlap) of the human KSR1 sequence were overlaid with recombinant GST-praja2 (1–531) followed by immunoblotting with anti-GST antibody. The sequences (in red) refer to the praja2-binding domain of KSR1. The amino acids methionine and cysteine have been substituted with alanine or serine, respectively. (e) Schematic representation of the peptides used for pull-down experiments (upper panel). Lysates from flag-praja2-transfected cells were subjected to pull-down experiment with GST or GST-KSR1pep carrying the praja2-binding domain fused to GST (lower panels). (f) Predicted structure of KSR1 kinase domain modeled on KSR2 template PDB 2Y4I. The position of the potential praja2-binding domain is indicated. (g) HEK293 cells were subjected to double immunostaining with monoclonal anti-KSR1 and polyclonal anti-praja2 antibodies. Images were collected and analyzed by confocal microscopy. Pearson's coefficients between praja2 and KSR1