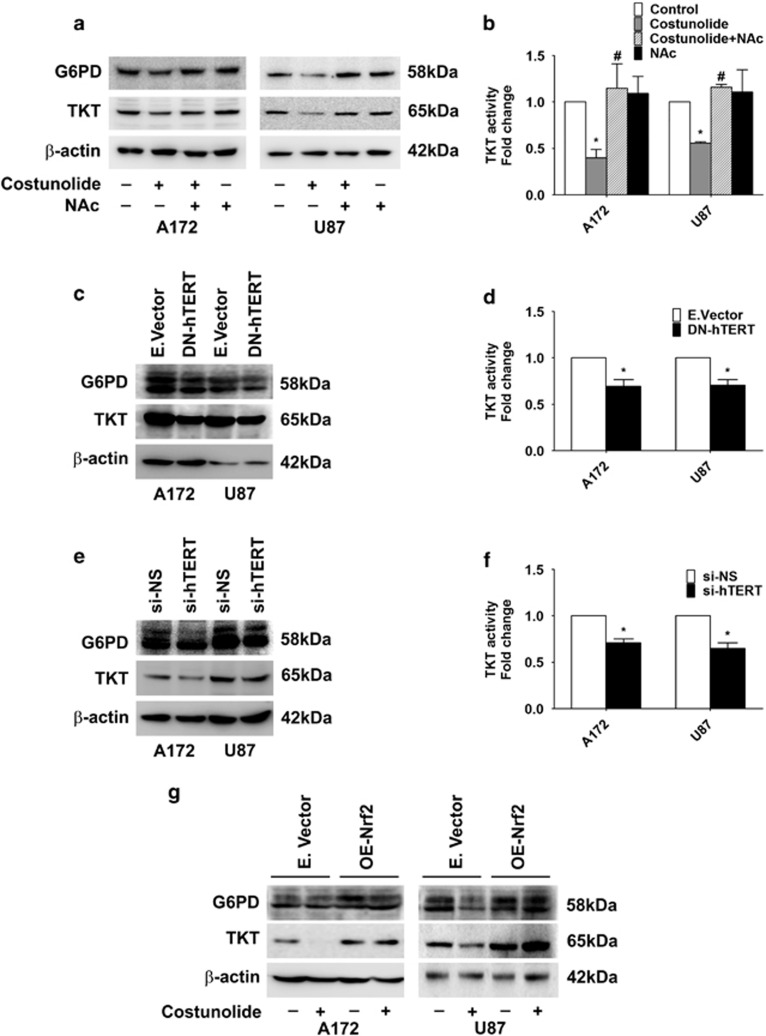
Figure 3.
TERT regulates pentose phosphate pathway in glioma cells. (a) Costunolide decreases expression of G6PD and TKT in an ROS-dependent manner. Western blot demonstrating G6PD and TKT levels in cells treated with different combinations of Costunolide and NAc. (b) Costunolide-mediated decrease in TKT activity is rescued upon ROS inhibition. Graph showing TKT activity in cells treated with Costunolide in the presence and absence of NAc. (c) Transfection of glioma cells with DN-hTERT decreases G6PD and TKT levels in glioma cells, as depicted by western blot analysis. (d) Decreased TKT activity in glioma cells transfected with DN-hTERT construct. Values represent fold change in TKT activity in DN-hTERT-transfected cells over mock-transfected control. (e) Western blot demonstrating decreased G6PD and TKT levels upon siRNA-mediated knock-down of hTERT. (f) siRNA-mediated knock-down of TERT decreases TKT activity in glioma cells. Values represent fold change in TKT activity in TERT siRNA-transfected cells over NS-siRNA-transfected control. (g) Western blot analysis depicting G6PD and TKT levels in glioma cells transfected with either Nrf2 overexpression construct (OE-Nrf2) or empty vector and treated in the presence or absence of Costunolide. Blots shown in (a, c, e, and g) are representative images of three independent experiments showing an identical trend. Blots were re-probed for β-actin to establish equivalent loading. Values in (b, d, and f) are means±S.E.M. of three independent experiments. *Denotes significant change control or mock-transfected group, #depicts significant change from Costunolide-treated cells (P<0.05)