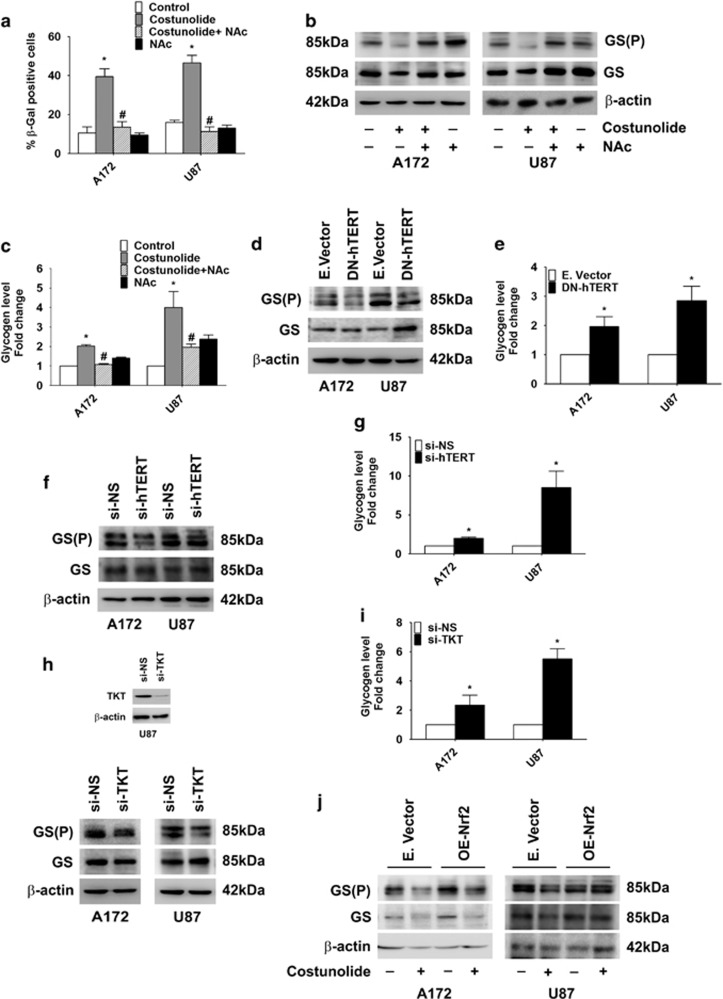
Figure 4.
TERT regulates glycogen accumulation and senescence. (a) Costunolide induces glioma cell senescence in an ROS-dependent manner. The graph represents percentage β-gal-positive cells observed upon treatment with Costunolide in the presence and absence of NAc. (b) Costunolide decreases phospho-glycogen synthase GS(P) levels in an ROS-dependent manner. Western blot depicting GS(P) and GS levels in cells treated with different combinations of Costunolide and NAc. (c) Costunolide-mediated increase in glycogen accumulation is ROS dependent. Graph showing glycogen levels in cells treated with Costunolide in the presence and absence of NAc. (d) Transfection with DN-hTERT diminishes GS(P) levels and (e) increases glycogen accumulation in glioma cells. (f) siRNA-mediated knock-down of TERT decreases phosphorylated GS levels and (g) increases glycogen accumulation. (h) Western blot analysis demonstrating diminished GS(P) levels in glioma cells upon siRNA-mediated knock-down of TKT. (i) siRNA-mediated knock-down of TKT increases glycogen accumulation. (j) Western blot analysis depicting the effect of Nrf2 overexpression on Costunolide-induced changes in GS(P) and GS levels in glioma cells. Blots (b, d, f, h, and j) are representative of three independent experiments showing similar results. Blots were re-probed for β-actin to establish equivalent loading. The values in the graph (a, c, e, g, and i) represent the mean±S.E.M. from three independent experiments. *Significant change from control or non-specific siRNA or mock-transfected cells, #significant change from Costunolide-treated cells (P<0.05)