Abstract
BACKGROUND
Human pregnancy requires robust hemostasis to prevent hemorrhage during extravillous trophoblast (EVT) invasion of the decidualized endometrium, modification of spiral arteries and post-partum processes. However, decidual hemorrhage (abruption) can occur throughout pregnancy from poorly transformed spiral arteries, causing fetal death or spontaneous preterm birth (PTB), or it can promote the aberrant placentation observed in intrauterine growth restriction (IUGR) and pre-eclampsia; all leading causes of perinatal or maternal morbidity and mortality. In non-fertile cycles, the decidua undergoes controlled menstrual bleeding. Abnormal uterine bleeding (AUB) accompanying progestin-only, long-acting, reversible contraception (pLARC) accounts for most discontinuations of these safe and highly effective agents, thereby contributing to unwanted pregnancies and abortion. The aim of this study was to investigate the role of decidual cells in uterine hemostasis, menstruation, inflammation, adverse pregnancy outcomes and abnormal uterine bleeding.
METHODS
We conducted a critical review of the literature arising from PubMed searches up to December 2015, regarding in situ and in vitro expression and regulation of several specific proteins involved in uterine hemostasis in decidua and cycling endometrium. In addition, we discussed clinical and molecular mechanisms associated with pLARC-induced AUB and pregnancy complications with abruptions, chorioamnionitis or pre-eclampsia.
RESULTS
Progestin-induced decidualization of estradiol-primed human endometrial stromal cells (HESCs) increases in vivo and in vitro expression of tissue factor (TF) and type-1 plasminogen activator inhibitor (PAI-1) while inhibiting plasminogen activators (PAs), matrix metalloproteinases (MMPs), and the vasoconstrictor, endothelin-1 (ET-1). These changes in decidual cell-derived regulators of hemostasis, fibrinolysis, extracellular matrix (ECM) turnover, and vascular tone prevent hemorrhage during EVT invasion and vascular remodeling. In non-fertile cycles, progesterone withdrawal reduces TF and PAI-1 while increasing PA, MMPs and ET-1, causing menstrual-associated bleeding, fibrinolysis, ECM degradation and ischemia. First trimester decidual hemorrhage elicits later adverse outcomes including pregnancy loss, pre-eclampsia, abruption, IUGR and PTB. Decidual hemorrhage generates excess thrombin that binds to decidual cell-expressed protease-activated receptors (PARs) to induce chemokines promoting shallow placentation; such bleeding later in pregnancy generates thrombin to down-regulate decidual cell progesterone receptors and up-regulate cytokines and MMPs linked to PTB. Endometria of pLARC users display ischemia-induced excess vasculogenesis and progestin inhibition of spiral artery vascular smooth muscle cell proliferation and migration leading to dilated fragile vessels prone to bleeding. Moreover, aberrant TF-derived thrombin signaling also contributes to the pathogenesis of endometriosis via induction of angiogenesis, inflammation and cell survival.
CONCLUSION
Perivascular decidualized HESCs promote endometrial hemostasis during placentation yet facilitate menstruation through progestational regulation of hemostatic, proteolytic, and vasoactive proteins. Pathological endometrial hemorrhage elicits excess local thrombin generation, which contributes to pLARC associated AUB, endometriosis and adverse pregnancy outcomes through several biochemical mechanisms.
Keywords: decidual cells, tissue factor, thrombin, contraception, uterine bleeding, pre-eclampsia, preterm birth
Introduction
Human reproduction is associated with significant risks of hemorrhage during blastocyst implantation and development of the primitive utero-placental lacunar circulation as well as subsequent co-option of the maternal blood supply via remodeling of spiral arteries and arterioles by endovascular trophoblasts and decidual natural killer (dNK) cells (Hanna et al., 2003; Macklon and Brosens, 2014). The latter immune system initiate the early steps of vascular remodeling by elaborating interferon-γ and angiogenic factors including vascular endothelial cell growth factor (VEGF)-C, the angiopoietins (Ang-1 and Ang-2) (Robson et al., 2012; Sharkey et al., 2015) and matrix metalloproteinases (MMPs), (MMP-2 and MMP-9) (Naruse et al., 2009). An even greater hemostatic burden is posed by parturition, when, following delivery, placental detachment and expulsion occurs. Conversely, the absence of pregnancy leads to the controlled bleeding of menstruation. Over the past 25 years, our laboratory has sought to increase understanding of these paradoxical physiologic properties of human endometrium by focusing on the elaboration of hemostatic, angiogenic and immuno-regulatory cytokines as well as proteases and their inhibitors by decidualized endometrial stromal cells.
In contrast with the physiological occurrence of menstruation, pathological bleeding into the decidualized endometrium of pregnancy triggers abruption [aka decidual hemorrhage]-associated preterm birth (PTB). Moreover, derangements in the expression of decidual cell-derived modulators of hemostasis, angiogenesis and immune response are implicated in the shallow placentation associated with abruptions as well as other adverse pregnancy outcomes including intrauterine growth restriction (IUGR), pre-eclampsia and idiopathic spontaneous PTB. Taken together these abnormal conditions are strongly associated with maternal and/or perinatal morbidity and mortality as well as enormous health care costs and are linked to accelerated rates of cardiovascular mortality later in life among affected mothers (Lykke et al., 2010b).
Progestin-only long-acting reversible contraceptives (pLARCs) are safe, highly effective and invaluable for use in women with preexistent thrombophilias in whom estrogen-containing agents are contraindicated. Administration of pLARCs is strongly associated with reduced rates of teenage pregnancy and abortion (Connolly et al., 2014; Secura et al., 2014). Moreover, pLARCs are relatively inexpensive making them ideal for use in less developed countries. However, pLARC-associated abnormal uterine bleeding (AUB) is generally not a health issue, but rather a source of personal inconvenience and annoyance and, in some societies, of religious taboo. It is the primary reason for discontinuation of these otherwise safe and effective agents (Short et al., 2014). Such AUB is linked to derangements in the physiological regulation of mediators of endometrial hemostasis, angiogenesis, vasoconstriction and inflammation.
Methods
This manuscript is a critical review of the literature arising from PubMed searches up to December 2015, regarding in situ and in vitro expression and regulation of several specific proteins involved in uterine hemostasis in decidua and cycling endometrium. In addition, we discussed clinical and molecular mechanisms associated with pLARC-induced AUB and pregnancy complications with abruptions, chorioamnionitis or pre-eclampsia.
Menstrual cycle physiology
Tissue factor expressed by decidualized endometrial stromal cells mediates uterine hemostasis
During a woman's reproductive years, ovarian derived circulating estradiol and progesterone induce cyclical changes in the endometrium in preparation for blastocyst implantation (Jabbour et al., 2006; Henriet et al., 2012). During the proliferative (follicular) phase of the menstrual cycle, rising circulating estradiol levels prime the endometrium for the actions of progesterone whose circulating levels are increased following ovulation and maintained at elevated levels during the secretory (luteal) phase to induce decidualisation of human endometrial stromal cells (HESCs). The decidualisation process is accompanied by marked changes in cellular differentiation; i.e. conversion of fibroblast-like HESCs into epithelial-like decidual cells surrounded by an extracellular matrix (ECM) enriched in basement-membrane-like proteins such as collagen IV and laminin (Wewer et al., 1985; Church et al., 1996; Iwahashi et al., 1996; Schatz et al., 1999). During decidualisation, more than 33 epigenetic effector genes are modulated in HESCs suggesting that complex epigenetic modification provide a wide range of plasticity required for transformation of HESCs to decidual cells (Grimaldi et al., 2012). Moreover, impaired decidualisation is strongly associated with recurrent pregnancy loss, suggesting the importance of proper decidual cell maturation in maintaining pregnancy (Salker et al., 2010; Lucas et al., 2015). Accompanying decidualisation, several key hemostatic proteins exhibit an increased expression in HESCs (Christian et al., 2001). Chief among these is tissue factor (TF), the primary initiator of coagulation (Lockwood et al., 1993a). TF is a 47 kDa trans-cell membrane glycoprotein member of the class-2 cytokine receptor family comprised of a hydrophilic extracellular domain, a membrane-spanning hydrophobic domain and a cytoplasmic tail. Under physiological conditions, endothelial cells do not express TF, whereas constitutive expression of TF in the adventitia of arteries and veins and in organ parenchymal cells forms a protective hemostatic envelope (Drake et al., 1989; Lockwood et al., 1993a; Mackman, 2004). As depicted in the coagulation cascade in Fig. 1, disruption of vascular integrity results in binding of cell membrane-bound TF to circulating plasma-derived factor VII or its active form, FVIIa. Consequently, factor Xa is generated directly by TF/factor VIIa, or indirectly via TF/VIIa activation of factor IX to IXa, which binds to its co-factor, factor VIIIa, to generate factor Xa. By either route, newly generated factor Xa binds to its co-factor factor Va to cleave prothrombin to thrombin (Bach, 1988). Once formed, thrombin converts fibrinogen to fibrin and activates platelets to promote hemostasis. The fibrin clot is then subjected to degradation by tissue type plasminogen activator (tPA) to restore vascular fluidity (Draxler and Medcalf, 2015). Premature fibrinolysis is prevented by plasminogen activator inhibitor type-1 (PAI-1), giving it a key role to play in modulating hemostasis (Sobel and Schneider, 2004).
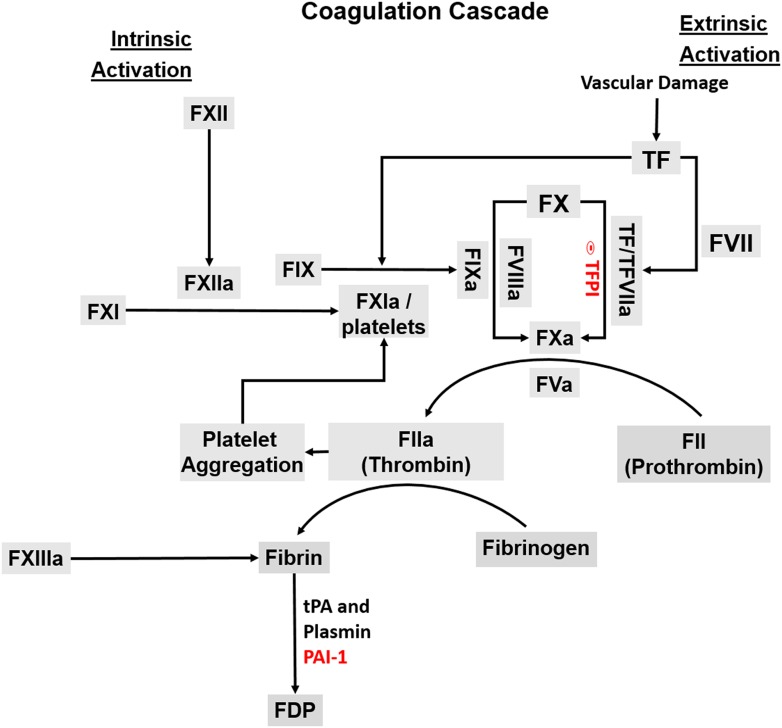
Figure 1.
Schematic demonstration of the coagulation cascade. The tissue factor (TF)–factor (F)VIIa complex of the extrinsic pathway initiates blood coagulation by activating factor (F) X directly or indirectly by activating FIX of the intrinsic pathway. Thrombin plays a pivotal role in this process by activating several proteases and cofactors. Specifically, thrombin cleaves fibrinogen to soluble fibrin monomers. These are cross-linked by FXIIa and thrombin activated platelets (not shown) to form the fibrin clot. Fibrin is degraded into the fibrin degradation products (FDP) by plasmin formed by tissue type plasminogen activator (tPA). The action of tPA is regulated by its fast inactivator, plasminogen activator inhibitor type (PAI)-1.
Thrombin also stimulates cells by binding to transmembrane protease-activated receptors (PARs). The PAR family is comprised of four members, PAR1, PAR2, PAR3 and PAR4. The expression of PARs on the surface of various cell types mediates several cellular effects including angiogenesis, cell proliferation and apoptosis, cytokine release and inflammation (Fu et al., 2015). For example, thrombin activation of PAR4 is implicated in promoting inflammation by affecting neutrophil recruitment, edema and plasma extravasation (Fu et al., 2015). In endometrial stromal cells, PAR1 activation increases expression of VEGF, MMPs, both tPA and urokinase plasminogen activator (uPA) as well as interleukin (IL)-8 and macrophage/monocyte chemoattractant protein (MCP)-1 to exert respective effects on angiogenesis and ECM degradation as well as the endometrial neutrophil and macrophage population (Osuga et al. 2012a).
Compensating for the absence of a canonical progesterone response element in the TF gene promoter, the induction of TF by progesterone in HESCs occurs via enhanced expression of the transcription factor, Sp1 (Krikun et al., 2000b) and requires the presence of epidermal growth factor (EGF) whose receptor is enhanced by progesterone in HESCs (Lockwood et al., 2000). The pivotal role played by HESC-expressed TF in reproductive hemostasis is underscored by observations that: (i) no human genetic TF deficiency has been described; and (ii) knocking out the TF gene results in abnormal yolk sac-derived circulation, hemorrhage and embryonic lethality by day 8.5 in mice (Carmeliet et al., 1996). Conversely, incorporation of a human TF mini-gene expressing 1% of wild type TF levels proved sufficient to rescue such TF knockout mice; although these low TF expressing mice when pregnant experience formation of placental labyrinth blood pools and high rates of lethal post-partum hemorrhage (Parry et al., 1998). Finally, progesterone stimulation of TF expression in HESCs continues throughout pregnancy protecting against bleeding; compared with the evanescent induction of the TF gene in all other reported tissues and cell types, this phenomenon represents a biologically unique effect (Lockwood et al., 2009). This sustained HESC expression of TF likely contributes to peripartum hemostasis.
Other factors produced by decidualized HESCs also promote endometrial hemostasis
Accompanying increased TF expression, decidualisation is also associated with enhanced HESC production of PAI-1, which acts as the primary anti-fibrinolytic agent by inhibiting the action of tPA, and also serves as an inhibitor of the ECM-degrading uPA (Schatz and Lockwood, 1993). Consistent with regulation of TF expression, the induction of PAI-1 gene transcription by progesterone is mediated by the Sp1 transcription factor and requires the presence of both EGF and its receptor (EGFR) (Lockwood et al., 2000; Lockwood, 2001; Krikun and Lockwood, 2002). Concomitantly, progesterone promotes stabilization of the endometrial vasculature by down-regulating HESC-derived MMPs, a family of zinc-requiring enzymes that includes collagenases, gelatinases and stromelysins (Lockwood et al., 1998, 2007a; Nagase and Woessner, 1999). Associated with the reduced expression of MMPs is down-regulation of PAs. The combined effects of these complementary changes inhibit fibrinolysis to prevent hemorrhage, and further stabilize the endometrial ECM to promote vascular integrity (Lockwood et al., 1995). Taken together, these coordinated progesterone-induced biochemical alterations create a local hemostatic endometrial environment that prevents potentially pregnancy terminating and life-threatening maternal hemorrhage during blastocyst implantation and placentation.
Menstruation is also mediated by HESCs
In non-fertile cycles, the premenstrual period of the late secretory (luteal) phase is accompanied by a decline in circulating progesterone levels, which triggers reductions in TF and PAI-1 expression (Lockwood et al., 1994b) to create a pro-hemorrhagic and fibrinolytic milieu. Concomitantly, progesterone withdrawal initiates intense vasospasm linked to the increased biological availability of epithelial-derived endothelin-1 (ET-1) and stromal cell-derived prostaglandin (PG) F2α synthesis, which elicit intense constriction of the spiral arterioles to produce hypoxia and generate vessel damaging reactive oxygen species (ROS) (Ohbuchi et al., 1995; Marsh et al., 1996; Salamonsen et al., 1999; Maybin et al., 2011b). In HESCs, declining progesterone levels also promote secretion of MMP-1, which preferentially degrades the fibrillar collagen MMP-3 which can initiate a local proteolytic cascade by degrading a wide array of ECM proteins and by activating the secreted forms of other MMPs, and PAs, which act in concert to promote sloughing of the functional endometrial layer by dissolution of the endometrial ECM during menstruation (Lockwood et al., 1995, 1998; Salamonsen et al., 1997; Schatz et al., 1994, 1997). Consistent with changes in human endometrium (Marbaix et al., 1992; Singer et al., 2000), up-regulation of several MMPs in response to progesterone withdrawal, have also been reported in both macaque and mouse models of menstruation (Rudolph-Owen et al., 1998; Kaitu'u et al., 2005; Critchley and Saunders, 2009; Coudyzer et al., 2013).
Endometrial post-menstrual repair and angiogenesis
Menstruation is followed by restoration of vascular integrity, angiogenesis and efficient endometrial repair (Okada et al., 2014; Maybin and Critchley, 2015). While rising estradiol levels stimulate proliferation of endometrial glandular and luminal epithelial cells as well as HESCs, vascular repair and angiogenesis is mediated by thrombin and/or hypoxia/ROS generated-VEGF derived from HESCs (Lockwood et al., 2002) and Ang-2, a pro-angiogenic vessel-branching and permeability factor (Albrecht and Pepe, 2003; Bonagura et al., 2010) derived from human endometrial endothelial cells (HEECs) (Krikun et al., 2000a). Simultaneously, the absence of progesterone and the presence of thrombin inhibit secretion of the blood vessel stabilizing and maturing agent, Ang-1 (Pfaff et al., 2006; Thomas and Augustin, 2009), derived from HESCs (Krikun et al., 2004). Other angiogenic factors are also induced in the endometrium during this period of endometrial repair. Specifically, in progesterone withdrawal, hypoxia and PGF2α act via hypoxia inducible factor (HIF)-1α and nuclear factor kappa B (NF-κB) transcription factors to induce expression of IL-8, a potent angiogenesis mediator and primary neutrophil chemoattractant (Tecchio and Cassatella, 2014; Tecchio et al., 2014) as well as adrenomedullin (AM), a pluripotent peptide member of the calcitonin gene peptide superfamily that induces vasodilation and proliferation of endothelial cells (Maybin et al., 2011a). These changes are accompanied by elevated endometrial expression of connective tissue growth factor (CTGF), a multifunctional growth factor and angiogenic agent expressed at high levels during wound repair and at sites of connective tissue formation (Igarashi et al., 1993; Brigstock, 2002; Ivkovic et al., 2003). Elevated CTGF mRNA and protein levels have been observed in post-menstrual endometrial epithelial cells and following treatment of endometrial epithelial cells with PGE2, PGF2α or hypoxia (Maybin et al., 2012).
Endometriosis and uterine hemostasis
Endometriosis is an inflammatory, estrogen-dependent gynecological disorder characterized by the presence of viable endometrial tissue, aka ectopic endometrial implants, outside the uterine cavity (Giudice and Kao, 2004; Krikun et al., 2008; Cakmak et al., 2009; Uz et al., 2011; Bourdel et al., 2015). In the USA, endometriosis affects ∼15% of all reproductive-aged women and 20–50% of infertile women (Krikun et al., 2008; Bulun, 2009; Han and O'Malley, 2014). Ectopic endometrial tissues are detected primarily on the pelvic peritoneum and ovarian surface and rarely in the pericardium, pleura, lung parenchyma, and even the brain (Krikun et al., 2008). The etiology of endometriosis reflects retrograde menstruation or coelomic metaplasia or differentiation of endometrial stem cells and/or embryonic duct remnants (Mai et al., 1998; Starzinski-Powitz et al., 2001; Taylor et al., 2002; Slater et al., 2005; Cakmak et al., 2009). Multiple cellular and molecular mechanisms including increased endometrial cell adhesion, angiogenesis, inflammation, an impaired immune response, aberrant estrogen signaling and progesterone resistance as well as reduced apoptosis are involved in the development, growth and survival of endometriotic lesions (Aznaurova et al., 2014; Taylor et al., 2015). However, the current review focuses on the roles of TF and thrombin in the pathogenesis of this disease.
An enhanced local inflammatory response is a common finding in both ectopic and eutopic endometrial tissues (Kayisli et al., 2007; Cakmak et al., 2009; Uz et al., 2011) with increased vascularization at the site of endometrial adhesion and subsequent invasion contributing to the survival and growth of ectopic endometrial implants. The role of TF as a well-defined inducer of angiogenesis (Ahamed and Ruf, 2004; Belting et al., 2004; Leppert and Eisenreich, 2015) stimulated our laboratory to compare TF expression in eutopic endometrium obtained from control women and from women with endometriosis as well as in ectopic endometrial loci using immunohistochemistry and in situ hybridization analyses. These studies revealed a progesterone-mediated prominent increase in TF mRNA and protein expression in decidualized HESCs during the luteal phase with glandular and luminal epithelium exhibiting minimal constitutive TF expression in normal endometrium throughout the menstrual cycle (Lockwood et al., 1993a, b; Schatz et al., 2003). In contrast, enhanced TF expression is observed in eutopic and ectopic endometrial glandular epithelial cells derived from women with endometriosis (Krikun et al., 2008). In addition to TF expression, thrombin receptors PAR-1 and PAR-2 were found to be highly up-regulated in the glandular epithelium of eutopic endometrium (Hirota et al., 2005; Krikun et al., 2008; Osuga et al., 2012b). Thus, local thrombin generation associated with retrograde menstruation may mediate endometriotic nidation and/or persistence. Taken together, these observations strongly support involvement of TF, thrombin and its receptors in mediating local angiogenic and inflammatory responses in endometriotic lesions (Fig. 2).
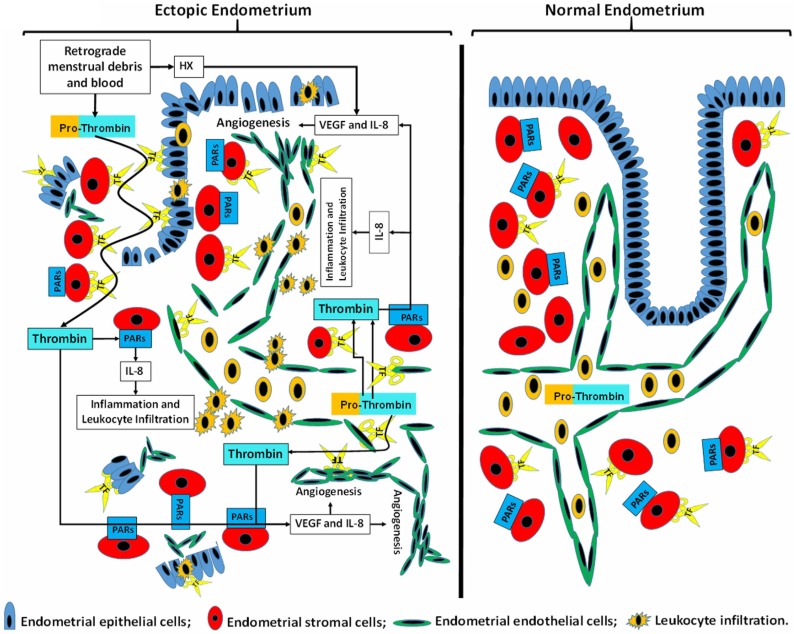
Figure 2.
Role of tissue factor generated thrombin in the pathogenesis of endometriosis. Expression of tissue factor (TF) is limited to endometrial stromal cells in normal endometrium, whereas in endometriosis, in addition to endometrial stromal cells TF is also expressed by epithelial and endothelial cells. TF expressed by stromal and epithelial cells generates excess thrombin by cleaving prothrombin present in retrograde menstrual cell debris and blood. Additional prothrombin cleavage occurs by TF expressed by endometriotic endothelial cells. The resulting excess thrombin binds to Protease-Activated Receptors (PARs) in endometriotic stromal cells to induce thrombin-mediated signaling cascades that initiate secretion of several cytokines and growth factors, specifically IL-8 and VEGF. Both cytokines trigger endometriotic angiogenesis, and IL-8 also recruits leukocytes, dominated by neutrophils. During retrograde menstruation, hypoxia also promotes vascularization of endometriotic implants. TF: tissue factor/factor VII/VIIa.
Under physiological conditions, TF is not expressed by the endothelium (Duanmu et al., 2011); however, endothelial cells of blood vessels in solid tumors and choroidal tissue in macular degeneration express endothelial TF (Contrino et al., 1996; Hu and Garen, 2000, 2001). Our laboratory described the anomalous expression of TF by endothelial cells in endometriotic lesions (Krikun et al., 2010b). The immunoconjugate molecule (Icon) is a complex of a mutated low-coagulation-inducing factor VII (fVII) domain that binds to TF with high affinity and an IgG1 Fc (fVII/IgG1 Fc) effector domain that activates a natural killer (NK) cell cytolytic response against TF-bearing endothelial cells (Hu et al., 1999; Hu and Garen, 2001). In view of high levels of TF in endometriotic endothelial cells, we used Icon in an athymic mouse model of endometriosis as a potential novel endometriosis therapy. These animal studies revealed that Icon administration disrupts vascular structures in endometriotic loci without apparent toxicity, reduced fertility and/or subsequent teratogenic effects to abolish endometriotic implants (Krikun et al., 2010b). The ability of Icon to eliminate pre-existing pathological vessels may provide a novel therapy in endometriosis. A recent study showed that the administration of ENMD-1068, a PAR-2 antagonist, significantly suppresses ectopic endometrial tissue growth in a mouse model revealing another potential novel therapy that targets TF and PAR signaling in treating endometriosis (Wang et al., 2014).
Aberrant placentation: role of decidual hemorrhage, inflammation and thrombin generation
Clinical observations
A retrospective cohort study of a large Danish pregnancy registry conducted by Lykke and colleagues determined that first trimester vaginal bleeding was most strongly associated with early (28–31 weeks) PTB (odds ratio [OR] 2.98; 95% CI: 2.50–3.54) (Lykke et al., 2010a). Lykke et al. also observed that such bleeding was associated with subsequent preterm premature rupture of the membranes (PPROM) (OR 1.18; 95% CI: 1.01–1.37), placental abruption (OR 1.48; 95% CI: 1.30–1.68), and severe pre-eclampsia (OR 1.25; 95% CI: 1.09–1.43). Prolonged vaginal bleeding for a minimum of 5 days in early pregnancy is associated with a 2-fold increase in risk of pre-eclampsia (North et al., 2011). Moreover, Weiss et al. (2004) found that that subjects experiencing light vaginal bleeding during the first trimester are more likely to have pre-eclampsia, preterm delivery, and placental abruption, whereas heavy vaginal bleeding subjects are more likely to experience intrauterine growth restriction, PTB, PPROM, and placental abruption. All of these disorders have been linked to shallow placentation, decidual vasculopathy and acute atherosis (Brosens et al., 2002, 2009; Veerbeek et al., 2015). Thus, the findings by Lykke et al. (2010a) indicate that first trimester bleeding is a marker of a primary defect in placentation. This hypothesis is supported by the work of Salafia and colleagues who observed increased prevalence of decidual hemosiderin in patients with PTB at less than 32 weeks (196/462; 43%) compared with those with term birth (1/108; 0.8%) (P < 0.00001) (Salafia et al., 1995). Moreover, decidual hemosiderin was also observed in 60% of patients with preterm pre-eclampsia (45/76), in 36% of patients experiencing preterm labor with intact membranes (58/161) and in 38% of patients with PPROM (72/192). This study by Salafia and colleagues also found that first trimester vaginal bleeding was commonly associated with such decidual hemosiderin deposition, suggesting that decidual hemorrhage was the primary cause of first trimester bleeding.
Molecular mechanisms of aberrant placentation
While the precise etiology of the aberrant/shallow placentation associated with these adverse pregnancy outcomes remains uncertain, decidual hemorrhage at any gestational age triggers substantial local thrombin generation derived from the abundant levels of TF expressed on decidual cell membranes. However, during the first trimester, but not at term, thrombin binds to PAR1 and PAR3 to increase expression of soluble fms-like tyrosine kinase-1 (sFlt-1) by cultured human decidual cells (Lockwood et al., 2007b). Other documented sources of enhanced sFlt-1 expression in pre-eclampsia include villous and extravillous trophoblasts (Tsatsaris et al., 2003; Nagamatsu et al., 2004; Fan et al., 2014) as well as maternal peripheral mononuclear cells (Rajakumar et al., 2005). A previous study showed that exogenous sFlt1 directly inhibits placental cytotrophoblast invasion in vitro (Zhou et al., 2002). Recent in situ observations revealed that lower levels of sFlt-1 immunoreactivity in placental villi are associated with invasive placentation suggesting a critical functional role for sFlt-1 in regulating placental invasion (McMahon et al., 2014). By irreversible binding to circulating VEGF, sFlt-1 blocks VEGF binding to its receptors on the endothelial cell membrane, thereby mediating VEGF signaling and its physiologic actions (Powe et al., 2011). During pregnancy, rodents overexpressing sFlt-1 develop hypertension, proteinuria and characteristic renal glomerular changes of endotheliosis that are clinical symptoms used to diagnose pre-eclampsia (Maynard et al., 2003). Therefore, such thrombin-induced decidual cell expressed sFlt-1 may promote shallow placentation by impeding local angiogenesis and extravillous trophoblast (EVT) invasion during early pregnancy. The specificity of these thrombin effects is evident since neither tumor necrosis factor-α (TNF-α) nor IL-1β, cytokines established to be associated with pre-eclampsia, IUGR and shallow placentation (Rinehart et al., 2001; Equils et al., 2005; Zhou et al., 2012; Matias et al., 2015), enhanced sFlt-1 expression in cultured first trimester human decidual cells (Lockwood et al., 2007b).
Previously, our laboratory demonstrated a statistically significant increase in macrophages (CD68-positive cells) and dendritic cells together with immunostaining of MCP-1, the primary monocyte recruiting and activating chemokine, in the decidua of preeclamptic patients (Lockwood et al., 2006b). Consequently, activated macrophages are associated with EVT apoptosis and impaired migration promoting shallow EVT invasion and impaired spiral artery remodeling linked to pre-eclampsia, abruption, stillbirth, IUGR and many cases of spontaneous PTB (Guenther et al., 2012; Melgert et al., 2012; Faas et al., 2014; Tang et al., 2015). Moreover, thrombin enhances the secretion of MCP-1 by primary cultures of first trimester human decidual cells (Matta et al., 2007). The pro-inflammatory cytokines, TNF-α and IL-1β also markedly increase MCP-1 secretion by cultured first trimester human decidual cells (Lockwood et al., 2006b). In addition, we observed that IL-1β and TNF-α significantly increase secreted levels of the specific macrophage activator, macrophage colony stimulating factor (M-CSF) by first trimester decidual cells and that conditioned medium supernatants from these cultures activate macrophages, which then promote caspase 3/7-dependent EVT apoptosis (Wu et al., 2012). Similarly, we observed increased expression of GM-CSF in preeclamptic decidua (Huang et al., 2010) and that TNF-α and IL-1β each markedly increase GM-CSF production by cultures of primary first trimester human decidual cells. Finally, incubation with IL-1β markedly increases decidual cell expression of five additional chemokines responsible for monocyte/macrophage chemoattraction and activation: C-C motif ligand 2 (CCL2), CCL5, C-X-C motif ligand 2 (CXCL2), CXCL3 and CXCL8 (Huang et al., 2006).
Role of immune cells in aberrant placentation
Several studies have shown the importance of regulatory T cells (Tregs) in physiopathology of pregnancy. During pregnancy, regulatory T cells (Tregs) play important roles in establishing and maintaining active immune tolerance (Aluvihare et al., 2004; Du et al., 2014). Tregs expand in the periphery and especially at the maternal-fetal interface in human and murine pregnancies. Moreover, the proportion of systemic and decidual Tregs (dTregs) was shown to be significantly lower in specimens from women with recurrent miscarriages compared with that in specimens from women with a normal pregnancy (Leber et al., 2010). Several studies implicated their deficiencies in pregnancy complications such as pre-eclampsia, but the results are inconsistent among studies (Quinn and Parast, 2013; Rahimzadeh et al., 2016). However, most recent studies suggest lack/reduced suppressive activity of Tregs in pre-eclampsia, associated with disruption of maternal immune tolerance (Hafeez et al., 2014; Boij et al., 2015; Nagayama et al., 2015).
Complementing increased numbers of activated macrophages in the decidua of patients with pre-eclampsia is a reciprocal reduction in numbers of dNK cells (Lockwood et al., 2013). In early human pregnancy, uterine spiral arteries and arterioles are converted to low resistance, high capacity vessels (conduits) that deliver increased blood flow required by the developing fetal-placental unit (Caniggia et al., 2000; Huppertz, 2014a; Salomon et al., 2014). This vascular conversion was initially attributed solely to fibrinoid-embedded blastocyst-derived EVTs (Robson et al., 2012). Subsequent studies indicated that dNK cells, accumulated around spiral arteries in the absence of EVTs (Bilinski et al., 2008; Male et al., 2010), initiate such early steps in vessel remodeling as vascular dilation, endothelial hyperplasia and vacuolization of medial smooth muscle cells by expressing MMPs (Smith et al., 2009), Interferon-γ (IFN-γ) and such angiogenic factors as VEGF-C and Ang-1 and Ang-2 (Ashkar and Croy, 2001; Kopcow and Karumanchi, 2007; Lash et al., 2010). By recruiting circulating NK cells to the decidua, trophoblasts (EVTs) may indirectly affect early vascular remodeling (Greenwood et al., 2000). Specifically, EVTs lining spiral arteries express stromal cell derived factor-1 (CXCL12), which binds to CCR4 expressed at the surface of the minority CD56brightCD16− circulating NK cell population to promote their recruitment (Hanna et al., 2003).
Studies in our laboratory revealed that co-incubation of primary cultures of human first trimester decidual cells with IFN-γ, a primary CD56bright16(−) NK cell product and either macrophage-derived TNF-α or IL-1β synergistically enhances mRNA and protein expression of IFN-γ induced protein 10 (IP-10) and IFN-inducible T-cell-α chemoattractant (I-TAC) via Janus kinase (JAK)/Signal Transducer and Activator of Transcription (STAT) and NF-κB signaling pathways (Lockwood et al., 2013). Both chemokines are documented to recruit CXCR3-expressing NK cells (Gasperini et al., 1999). Consistent with this in vitro action, immunostaining of first trimester decidua showed expression of IP-10, I-TAC, IFN-γ receptor 1 and 2, which are required to mediate the actions of IFN-γ on decidual cells (Chen et al., 2015).
Flow cytometry identified elevated CXCR3 expression on dNK cells and on the minority of circulating peripheral CD56brightCD16− NK cells and intermediate CXCR3 expression on the majority circulating CD56dimCD16+ NK cells. Peripheral NK cells incubated with either IP-10 or I-TAC displayed a concentration-dependent increase in CXCR3 levels and enhanced NK cell migration that peaked at 10 ng/ml. However, higher IP-10 and I-TAC concentrations (>50 ng/ml) elicited down-regulation of NK cell CXCR3 expression and migration. Consistent with this biphasic in vitro action, decidua derived from women with pre-eclampsia displayed significantly lower NK cell numbers and higher IP-10 and I-TAC expression compared with gestational age-matched controls. Consistent with these observations, we found significantly elevated IP-10 levels in first trimester sera from women destined to develop pre-eclampsia (Lockwood et al., 2013). A recent study from our laboratory demonstrated that first trimester decidual cells are also a significant source of IFN-γ (Chen et al., 2015). Thus, immunostaining of first trimester decidual sections revealed that both decidual cells and dNK cells express IFN-γ. However, while individual NK cells express higher levels of IFN-γ, the more numerous decidual cells account for a greater proportion of total decidual IFN-γ immunostaining. These findings suggest a ‘Goldilocks’ phenomenon in which some inflammation is required for optimal dNK and EVT remodeling of decidual and myometrial spiral arteries to increase uteroplacental blood flow, but excess inflammation may promote shallow placentation.
The cytokine, IL-11 is also a hemopoietic growth factor that exhibits pleiotropic biological effects, including induction of T helper cell type 2 (Th2) and inhibition of T helper cell type 1 (Th1) cytokine responses as well as induction of decidua-specific maturation of NK cells (Quesniaux et al., 1992; Weich et al., 1997; Bozza et al., 2001; Curti et al., 2001; Cakmak et al., 2005), indicating complex regulatory effects in controlling inflammation. Acting as an anti-inflammatory agent, IL-11 reduces TNF-α levels in endometrial epithelial cells (Cork et al., 2002) as well as IL-1β, TNF-α, IL-12, and nitric oxide production in activated macrophages (Trepicchio et al., 1996) and CD4+ T cell production of T helper cell type 1 cytokines, such as IL-12 induced IFN-γ, while enhancing the expression of Th2 cytokines, such as IL-4 and IL-10 (Bozza et al., 2001; Curti et al., 2001). However, its well-documented pro-inflammatory properties include induction of PGE2 secretion during bone resorption (Morinaga et al., 1998; Morgan et al., 2004) and stimulation of the expression of several acute phase proteins, including fibrinogen, C-reactive protein, ferritin, and haptoglobin (Gordon et al., 1996; Morinaga et al., 1998). In addition to changes in IP-10 and ITAC expression, we detected a pre-eclampsia-related increase in IL-11 levels in human decidual cells, but no changes in interstitial trophoblast at the maternal-fetal interface (Basar et al., 2010). In parallel with these findings, IL-1β and TNF-α significantly induced levels of IL-11 mRNA expression and protein secretion in first trimester decidual cell cultures. Both the IL-1β- and TNF-α-mediated increases in IL-11 levels were blunted by specific inhibitors of the p38 mitogen-activated protein kinase (MAPK) and NF-κB, but not by protein kinase C signaling pathways (Basar et al., 2010). As noted above, the role of IL-11 in regulating Th2 and Th1 cell activity and maturation of dNK cells as well as the complex involvement of IL-11 expression with inflammation, decidualization and trophoblast invasion suggests that enhanced expression of IL-11 contributes to the pathogenesis of pre-eclampsia by impairing dNK cell-mediated induction of endovascular EVT invasion of the decidua, the primary placental defect of pre-eclampsia.
Previously, our laboratory demonstrated higher immunostaining for MMP-9 in decidual cells of preeclamptic compared with gestational age-matched control placentas and that TNF-α and IL-1β each induce expression of MMP-9 mRNA and protein in cultured first trimester decidual cells (Lockwood et al., 2008). We also observed that p38 MAPK signaling mediates TNF-α enhancement of first trimester decidual cell MMP-1, MMP-3, and MMP-9 expression (Lockwood et al., 2014). These MMPs display both individual and overlapping ECM-degrading activities. Specifically, interstitial collagenase (MMP-1) preferentially degrades interstitial and fibrillar collagens, and stromelysin (MMP-3) degrades a broad array of proteins and can initiate a proteolytic cascade by activating the secreted zymogenic form of other MMPs such as pro-MMP-1 and pro-MMP-9, a collagenase (Itoh and Nagase, 1995; Westermarck and Kahari, 1999; Lijnen, 2002; Cohen et al., 2006; Nagase et al., 2006). During placentation, an inflammatory cytokine-induced MMP overexpression initiates a proteolytic cascade that disrupts the decidual ECM structure/integrity to interfere with normal stepwise integrin-mediated EVT invasion of the decidua and associated spiral artery and arteriole remodeling (Damsky and Fisher, 1998; Lyall, 2005; Pijnenborg et al., 2006). Unexpectedly, our laboratory observed that IFN-γ inhibits this TNF-α enhancement of decidual cell p38 MAPK phosphorylation to block increased MMP-1, 3, and 9 expression, thereby stabilizing the decidual ECM to protect against pre-eclampsia (Lockwood et al., 2014). Taken together, these findings also suggest the existence of a delicate balance that enables decidual cell and dNK cell-derived IFN-γ to synergistically interact with low levels of decidual macrophage-derived TNF-α to chemoattract peripheral CD56bright/CD16− NK cells to the decidua. These newly recruited dNK cells can then release additional IFN-γ to promote EVT invasion, stabilize the decidual ECM and facilitate spiral artery vascular remodeling. Supporting these observations, IFN-γ deficient pregnant mice fail to display characteristic mid-gestational decidual vascular dilation and loss of smooth muscle. However, treatment with IFN-γ ameliorates these effects and mediates transformation of uterine spiral arteries to low resistance, high capacity vessels (Monk et al., 2005). In contrast to this physiological state, pre-conceptional or peri-conceptional decidual inflammatory states associated with increased TNF-α or IL-1β generation would predictably upset this delicate balance to recruit to the decidua activated monocyte/macrophages whose elaboration of excess TNF-α causes EVT apoptosis, restrains dNK cell chemotaxis and disrupts decidual ECM to promote shallow placentation associated with various adverse pregnancy outcomes. Such a decidual inflammatory state could be caused by a variety of potential mechanisms including nulliparity-associated exaggerated alloimmune responses, autoimmune dysfunction, anti-phospholipid antibodies, severe obesity, chronic microbial infections and/or excess decidual thrombin generated by focal hemorrhage or severe thrombophilic conditions (e.g. anti-thrombin deficiency).
Decidual hemorrhage and inflammation are major contributors to preterm birth
Clinical observations
The occurrence of decidual hemorrhage and inflammation in the first trimester of pregnancy are strongly linked to aberrant placentation (Orsi and Tribe, 2008; Huppertz, 2014b). Moreover, bleeding into the decidua (i.e. abruption) (Naeye, 1983; Salafia et al., 1995; Ananth et al., 2007) and the occurrence of decidual inflammation later in pregnancy are strongly associated with spontaneous PTB (Romero et al., 1988, 2002; Yoon et al., 2001; Shim et al., 2004). These two phenomena are linked since abruption (i.e. bleeding into the decidua basalis) is commonly (58%) associated with incomplete spiral artery transformation (Oyelese and Ananth, 2006). Abruption may present as vaginal bleeding, retrochorionic hematoma formation or a concealed abruption. The latter, if undermining >50% of the overlying placenta, can result in fetal death (Oyelese and Ananth, 2006). Vaginal bleeding in more than one trimester is associated with a 7-fold increased risk of PPROM and PTB compared with the absence of bleeding (Harger et al., 1990), complementing the particularly strong association between abruption and PTB due to either PPROM or preterm labor with intact membranes between 22 and 32 weeks (Salafia et al., 1995). Manuck and associates analyzed results from a multicenter, prospective study of 1025 women having singleton gestations with spontaneous PTB <34 weeks to categorize their clinical phenotype (Manuck et al., 2015). This study reported decidual hemorrhage in 39% of women with very early (20–28 weeks) PTB, whereas decidual hemorrhage was present in 28% of cases of PTB between 28 and 34 weeks.
Abruptions are associated with significant thrombin generation manifested by the common occurrence of co-existent hypofibrinogenemia and frank consumptive coagulopathy. Previously, our laboratory found that thrombin activation, as measured by elevated second trimester plasma thrombin-anti-thrombin (TAT) levels, predicts the subsequent occurrence of PPROM and PTB with high sensitivity and specificity (Elovitz et al., 2001; Rosen et al., 2001; Chaiworapongsa et al., 2002).
Molecular mechanisms underlying thrombin-induced abruption
We have examined mechanisms underlying the strong association between decidual hemorrhage and PPROM and observed that thrombin-PAR interactions lead to the release of active MMP-1 and -3 by term decidual cells (Rosen et al., 2002; Mackenzie et al., 2004). Thrombin exhibits concentration-dependent direct weakening of isolated amniotic membranes and promotes amniotic MMP-9 release. All three MMPs are integral to fetal membrane rupture and cervical change (Fortunato et al., 1999; Maymon et al., 2000; Becher et al., 2008; Zaga-Clavellina et al., 2011). A recent study (Mogami et al., 2014) revealed that thrombin activity is increased in PTB-derived human amnion and that thrombin treatment enhances MMP-1 and MMP-9 mRNA levels and enzymatic activity as well as cyclooxygenase 2 (COX-2) mRNA and PGE2 production in amniotic mesenchymal, but not epithelial, cells. Moreover, this study localized PAR-1 to amniotic mesenchymal cells and decidual cells and demonstrated that thrombin-induced up-regulation of MMP-9 is specifically mediated by PAR-1, whereas thrombin enhanced MMP-1 and COX-2 expression is mediated via Toll-like receptor-4. In pregnant mice, thrombin injection induces PTB. Taken together, these observation indicate that thrombin acts through distinct mechanisms to activate MMPs and PGE2 synthesis in amnion, thereby, contributing to PTB (Mogami et al., 2014). Our group also demonstrated that abruptions are associated with a marked increase in decidual neutrophil infiltration accompanied by neutrophil co-localization with fibrin deposition, which peaks after PPROM. In contrast, decidua from gestational age-matched controls is virtually devoid of neutrophils. Moreover, thrombin markedly enhances mRNA and protein levels of the primary neutrophil chemoattractant IL-8 (Bergin et al., 2010) in term decidual cells (Lockwood et al., 2005).
In sections of human decidua, we found that abruption-associated PTB is accompanied by a decrease in immunohistochemical staining for decidual cell progesterone receptor (PR) expression (Lockwood et al., 2012). Treatment of primary third trimester decidual cell cultures with thrombin elicited a concentration-dependent reduction in the two nuclear PR protein isoforms, PR-A and B and PR mRNA levels, mediated by phosphorylation of the signal transduction mediator, ERK1/2. Moreover, immunostaining for phospho-ERK1/2 was increased in decidual cells derived from abruption versus control decidua. Abruption-associated-reduced PR-A and PR-B expression by decidual cells suggests a mechanism by which excess thrombin derived from decidual cell-expressed TF acts as an autocrine/paracrine inducer of functional progesterone withdrawal (Zakar and Hertelendy, 2007; Patel et al., 2015) to elicit abruption-related PPROM and PTB (Fig. 3).
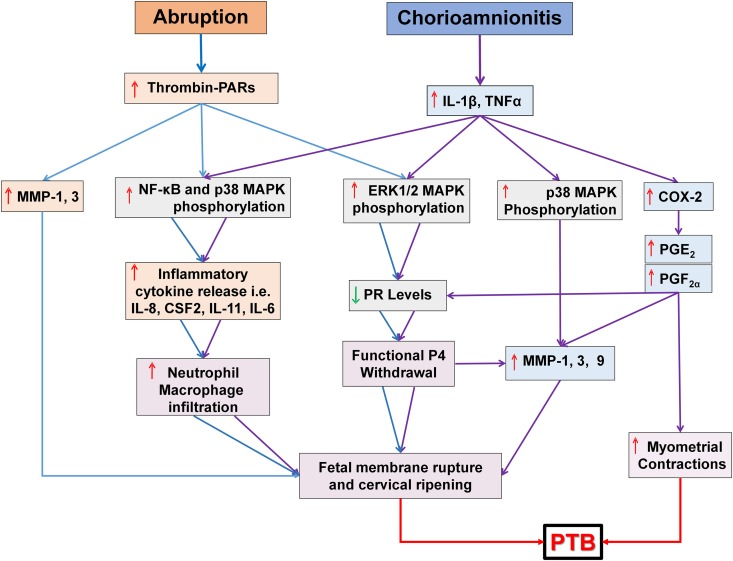
Figure 3.
Cellular and molecular mechanisms leading to preterm birth. In the presence of decidual hemorrhage, thrombin generated from decidual cell-expressed TF activates NF-κB and p38 MAPK signaling pathways. Both pathways enhance production of several cytokines (IL-8, IL-11, CSF2, etc.), which recruit neutrophils that elaborate various matrix metalloproteinases (MMPs) as well as other proteinases. Thrombin directly enhances MMP-1 and -3 production in decidual cells. Collectively, these proteinases promote fetal membrane rupture and cervical change. Thrombin also suppresses decidual cell progesterone receptor (PR) levels by increasing ERK1/2 phosphorylation. The resulting functional progesterone withdrawal in the decidua further induces MMP expression and decidual inflammation. In the case of chorioamnionitis, pro-inflammatory mediators (IL-1β or TNF-α) and/or bacterial products (e.g. endotoxins) activate NF-κB, ERK1/2 and p38 MAPK mediated signaling pathways in decidual cells. NF-κB and p38 MAPK activation enhances production of cytokines (IL-8, IL6, IL11, etc.) and expression of MMP-1, -3, and -9 as well as cyclooxygenase (COX2), which in turn induce prostaglandins (PG) production in decidual cells. Moreover, the activated ERK1/2 MAPK signaling cascade lowers PR isoform levels causing functional progesterone withdrawal. Taken together, functional progesterone withdrawal, MMPs and increased PG production trigger uterine contractions, fetal membrane rupture and cervical ripening leading to chorioamnionitis-associated preterm birth (PTB).
In the previously cited study by Manuck et al., inflammation and infection were found to be common causes of PTB (Manuck et al. 2015). Their study observed that among women with early (28–34 weeks) and very early (20–28 weeks) PTB, clinical and/or histological evidence of chorioamnionitis was present in 35 and 47% of cases, respectively. Analogous to the parallel effects of thrombin and either, TNF-α or IL-1β on first trimester decidual cells leading to aberrant placentation, TNF-α and IL-1β are integral to chorio-decidual infection (Flores-Herrera et al., 2012) with both cytokines exhibiting similar in vitro pro-parturition effects on third trimester human decidual cells as those displayed by thrombin. Moreover, as is the case for abruption-related PTB, decidual cell immunostaining for MMP-1 and -3 is greatly increased in chorioamnionitis-complicated preterm decidual sections compared with control sections (Oner et al., 2008). In third trimester human decidual cell cultures, TNF-α significantly enhances MMP-1 and -3 release by 14 ± 3 and 9 ± 2-fold, respectively, while IL-1β exerts even greater effects by increasing MMP-1 and -3 release, 13 ± 3- and 19 ± 2-fold, respectively. All of these effects are mediated by activation of p38 MAPK signaling (Fig. 3). We also observed that while neither TNF-α nor IL-1β weakened isolated amniotic membranes, co-incubation of either of these cytokines with both amnion side (facing the fetal site) and choriodecidua side (facing the maternal side) or with full thickness fetal membranes resulted in weakened fetal membranes together with excess production by the choriodecidua of MMP-9. These findings underscore the crucial role of decidual cells in mediating inflammation-associated PPROM (Kumar et al., 2011). Extending these observations, a recent study by Kumar et al. demonstrated that weakening of full-thickness fetal membrane fragments exposed to TNFα or thrombin is blocked by pre-incubation with a CSF-2 antibody. A concurrent elevation of CSF-2 levels on the choriodecidua, but not the amnion side suggest that CSF-2 is a critical common intermediate in both thrombin derived from decidual cell-expressed TF and TNF-α induced fetal membrane weakening (Kumar et al., 2014).
Molecular mechanisms contributing to inflammation-induced preterm birth
As is the case with abruption-associated PPROM, chorioamnionitis-associated PTB is accompanied by intense neutrophil infiltration of the decidua. We found that decidua from chorioamnionitis-complicated pregnancies, but not from term controls, displayed marked immunohistochemical staining for IL-8, as noted above, the primary neutrophil chemoattractant (Rosenkilde and Schwartz, 2004; Dimberg, 2010; Gales et al., 2013) in decidual cells accompanied by dense decidual neutrophil infiltrates (Lockwood et al., 2006a). In third trimester leukocyte-free decidual cell cultures, TNF-α and IL-1β induced a respective 236.6 ± 51.4 and 1062.6 ± 254.3-fold release of IL-8 accompanied by a corresponding 10- and 100-fold increase in steady state IL-8 mRNA levels (Fig. 3). Similarly, our laboratory demonstrated a marked increase in immunostaining for CSF2, (aka GM-CSF), which is a potent neutrophil and monocyte/macrophage chemoattractant and activator (Castagnola and Dufour, 2014; Hansen et al., 2014), in the decidua of patients with chorioamnionitis compared with controls (Arcuri et al., 2009). Augmenting these in situ observations, human third trimester leukocyte-free decidual cells treated with TNF-α or IL-1β exhibited statistically significant 18- and 245-fold increases in CSF2 protein release, respectively, accompanied by parallel changes in CSF2 mRNA levels.
In contrast with the low neutrophil numbers in normal human decidua, neutrophils are the dominant immune cell type in the decidua in most cases of chorioamnionitis-induced PTB (Bry and Hallman, 1989; Presicce et al., 2015). The maternal origin of these decidual neutrophils in cases of PTB associated with acute chorioamnionitis was demonstrated by fluorescence in situ hybridization for X and Y chromosomes (Steel et al., 2005). However, a subset of cases of chorioamnionitis linked to both neonatal intraventricular hemorrhage and death display a predominance of decidual monocytes/macrophages (Andrews et al., 2006). Our laboratory demonstrated significantly higher decidual cell immunostaining for IL-6, a primary monocyte chemoattractant (Jones, 2005; Medzhitov, 2008; Schaper and Rose-John, 2015), in chorioamnionitis-associated PTB compared with gestational age-matched control placental specimens (Lockwood et al., 2010). Levels of IL-6 were markedly increased in leukocyte-free term decidual cells by 2500-fold during incubations with IL-1β. Corresponding changes were also noted for steady state levels of IL-6 mRNA. Specific inhibitors of signaling for both NF-κB activation and p38 MAPK, but not for protein kinase C, inhibited this IL-1β inductive effect. Cervical IL-6 levels greater than 250 pg/ml between 24 and 36 weeks predict subsequent PTB with a sensitivity of 50.0% (95% CI: 33.2–66.8%) and a specificity of 85.0% (95% CI: 78.8–91.2%) (Lockwood et al., 1994a), a predictive value only modestly inferior to fetal fibronectin (Lockwood et al., 1991). These studies were extended to include IL-11, which is a key member of IL-6 cytokine family. Specifically, we observed significantly higher IL-11 immunostaining in decidual cells at the maternal-fetal interface obtained from women following preterm versus term delivery. These in situ observations were complemented by a marked increase in IL-11 production in term decidual cell cultures in response to thrombin or IL-1β treatment (Cakmak et al., 2005). These findings suggest that aberrant IL-11 expression at the maternal-fetal interface is involved in inflammation- and abruption-associated prematurity since excess thrombin generation and IL-1β levels are associated with abruption- and chorioamnionitis-related preterm deliveries, respectively.
Analogous with abruption-associated PTB, immunostaining of human decidual sections identified significantly lower PR levels in decidual cells from chorioamnionitis-complicated PTB compared with gestational age matched controls (Guzeloglu-Kayisli et al., 2015b). In correspondence with these in situ observations, treatment of third trimester leukocyte-free decidual cells with IL-1β significantly reduced PR mRNA and protein expression while significantly enhancing decidual cell PGE2 and PGF2α production and COX-2 expression. We also found that PGF2α, but not PGE2, suppressed decidual cell PR expression. However, the COX inhibitor, indomethacin, failed to reverse IL-1β inhibition of decidual cell PR expression, indicating that this cytokine directly inhibits PR expression, and therefore, that this suppression does not reflect a PGF2α autocrine effect. While IL-1β treatment of decidual cells activated NF-ĸB, ERK1/2 and p38 MAPK signaling cascades, inhibition of ERK1/2 alone reversed IL-1β suppression of PR levels (Fig. 3). Thus, both thrombin and IL-1β inhibit expression of nuclear PR-A and PR-B to induce functional progesterone withdrawal via the same signal transduction pathway, demonstrating a parsimonious final common mechanism for abruption and infection-associated PTB (Lockwood et al., 2012; Guzeloglu-Kayisli et al., 2015b).
Abnormal uterine bleeding associated with pLARCs
Clinical observations
Administration of pLARCs to women results in safe, effective, and discrete long-term contraception (Affandi, 1998; Kaunitz, 2000; Rafie et al., 2014). Currently available forms include: (i) Depo-Provera, an injectable form of medroxyprogesterone acetate (MPA) lasting 3 months; (ii) Mirena® intrauterine system (IUS) which releases levonorgestrel (LNG) for 5 years; (iii) Skyla IUS, a smaller version of Mirena with lower LNG content, ideally suited for nulliparous women and lasting over 3 years; and (iv) Nexplanon a single-rod etonogestrel contraceptive placed subdermally in the inner upper arm for 3 years (Varney and Guest, 2004; Chaovisitsaree et al., 2005; Mutihir and Daru, 2008). Nexplanon is radio-opaque for ease of identification and removal. The extremely effective contraceptive properties of these agents reflect changes in cervical mucus and tubal motility that impair sperm migration, inhibition of gonadotrophin secretion to block ovulation, and, potentially, establishment of a hostile endometrial environment to prevent blastocyst implantation (Birgisson et al., 2015). These agents are safe to use during lactation and are particularly appropriate to administer to women in whom estrogen therapy is contraindicated due to increased thromboembolic risks (Kaunitz, 2000). A large prospective cohort study in which more than 75% of teenage participants received pLARCs found a substantial reduction in mean annual rates of pregnancy, birth, and abortion compared with the overall pool of sexually experienced US teens (Secura et al., 2014).
The occurrence of abnormal uterine bleeding in the majority of pLARC users is a major reason for non-adherence. Three year discontinuation rates were 47% for implant and 27% for IUS users, respectively (Weisberg et al., 2014). Unlike the predictable and controlled nature of menstrual bleeding, pLARC-associated AUB occurs unpredictably and sporadically.
Cellular and molecular mechanisms underlying pLARC-induced abnormal uterine bleeding
We have biopsied bleeding and non-bleeding endometrial sites in pLARC versus secretory phase controls and observed that such AUB arises from superficial, enlarged, thin-walled fragile vessels denuded of pericytes and vascular smooth muscle cells, embedded in a collapsed stromal ECM (Runic et al., 2000; Kayisli et al., 2015). Unexpectedly, after 1 year of pLARC treatment, immunostaining demonstrated that TF levels are increased at endometrial bleeding versus non-bleeding sites. Equally surprising, bleeding sites exhibited high HESC immunoreactive PR and EGFR levels, suggesting that the molecular mechanisms responsible for pLARC-associated bleeding differed from those found in progesterone withdrawal triggered menstrual bleeding. This led us to examine the genesis of the abnormal endometrial vasculature found in these patients. We observed that pLARC treatment reduced endometrial blood flow up to 8-fold (Fig. 4) as measured by laser Doppler fluxmetry (Hickey et al., 2006b). Studies in an animal model indicate that this effect is likely due to direct progestin-mediated vasoconstrictive effects on uterine or radial arteries (Krikun et al., 2012). Compatible with this observation, pLARC treated endometria displayed evidence of hypoxia/reperfusion injury. Thus, women treated with the pLARC, Implanon, displayed increased endometrial immunostaining for the phosphorylated forms of the stress-activated kinases SAPK/JNK and p38 MAPK, markers of hypoxic injury (Krikun et al., 2002). Consistent with these in situ observations, hypoxia and ROS induced phosphorylation of SAPK/JNK and p38 MAPK in primary cultures of both HESCs and HEECs. The endometria of pLARC users also display evidence of excess ROS generation (Fig. 4), as indicated by increased staining for nitrotyrosine and 8-hydroxydeoxyguanosine as well as evidence of increased 8-isoprostane production, a marker of lipid peroxidation (Hickey et al., 2006b).
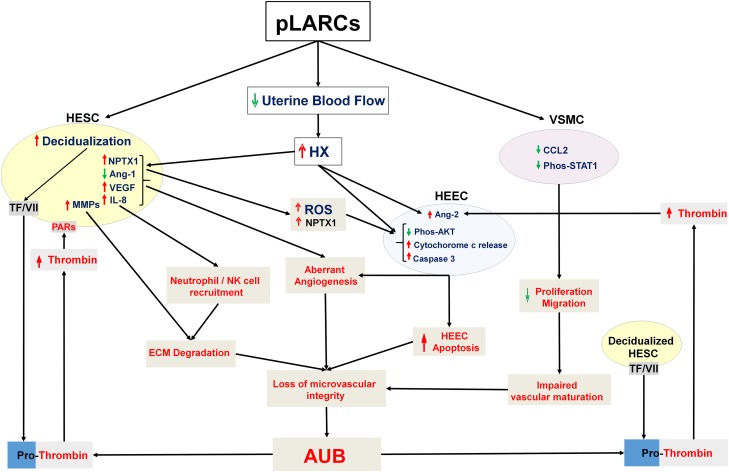
Figure 4.
Cellular mechanisms of AUB in progestin-only, long-acting, reversible contraception (pLARC). Administration of pLARCs induces human endometrial stromal cell (HESC) decidualization and reduces endometrial blood flow causing local hypoxia. In decidualized HESCs, this hypoxia inhibits expression of angiopoietin (Ang)-1 while increasing expression of vascular endothelial growth factor (VEGF), interleukin (IL)-8 and matrix metalloproteinases (MMPs). In human endometrial epithelial cells (HEECs), hypoxia and ROS increase expression of Ang-2, a potent angiogenic agent. Hypoxia and reperfusion generate reactive oxygen species (ROS) that directly damages blood vessels, resulting in enhanced vascular permeability. The latter delivers circulating factor VII to HESC membrane bound tissue factor (TF), which activates factor Xa to generate thrombin. The resulting excess thrombin exacerbates hypoxia/ROS effects on endometrial angiogenesis and inflammation. pLARCs-induced hypoxia/ROS enhances HESC secreted NPTX1 which trigger HEEC apoptosis by inhibiting AKT signaling, causing mitochondrial dysfunction, enhancing cytochrome c release, and activating a caspase 3 mediated cascade. Moreover, pLARCs directly inhibit vascular smooth muscle cells (VSMC) proliferation and migration by blocking CCL2-mediated STAT1 signaling. The combined impact of these molecular mechanisms promotes aberrant angiogenesis and endothelial apoptosis, and reduces numbers of VSMCs to generate thin-walled hyperdilated fragile vessels prone to leakage and bleeding.
Consistent with the effect of hypoxia as the prime driver of angiogenesis (Hashimoto and Shibasaki, 2015; Schonenberger and Kovacs, 2015; Zimna and Kurpisz, 2015), histological sections of endometria from pLARC-treated patients display expected reduced immunostaining for Ang-1, as previously noted, a vessel stabilizing factor (Fukuhara et al., 2010; Koh, 2013), and increased expression of the primary mediator of angiogenesis, VEGF, in HESCs with both changes correlating positively with microvascular density and increased bleeding profiles (Lau et al., 1999; Roopa et al., 2003; Lockwood et al., 2004b; Pritts et al., 2005). Simultaneously, increased immunostaining for Ang-2, a pro-angiogenic vessel-branching and permeability factor (Thurston and Daly, 2012; Eroglu et al., 2013; Gerald et al., 2013), was evident in HEECs (Lockwood et al., 2004b). Furthermore, while MPA enhanced Ang-1 protein and mRNA expression, both hypoxia and ROS markedly decreased Ang-1 expression in HESCs (Krikun et al., 2002). Conversely, HEECs maintained under hypoxia exhibited significantly increased Ang-2 expression (Fig. 4).
Excess thrombin is expected to be generated in response to pLARC-associated AUB. Thus, we incubated primary cultures of HESCs and human endometrial glandular epithelial cells (HEGECs) with MPA ± thrombin under hypoxia or normoxia. Compared with normoxia, hypoxia enhanced secreted levels of VEGF several-fold in HESCs or HEGECs, whereas MPA did not significantly affect VEGF output by either cell type under normoxia or hypoxia. Addition of thrombin to cultured HESCs elicited significant concentration and time-dependent increases in VEGF protein and mRNA that peaked at 48 h. Multiple VEGF isoforms were enhanced in HESCs by thrombin including VEGF-121, -165 and -189. By contrast, HEGECs were unresponsive to thrombin added either alone or with MPA. Extrapolation of these in vitro findings to endometria from pLARC treated women suggests that bleeding into decidualized HESCs that over-express TF generates thrombin, which in turn acts as an autocrine potentiator of VEGF expression that exacerbates abnormal endometrial angiogenesis found in these women (Fig. 4) (Lockwood et al., 2002).
To account for the fragility of endometrial microvessels in pLARC-administered women, we hypothesized that paracrine factors from pLARC-treated HESCs promote HEEC apoptosis. In support of this hypothesis, conditioned media supernatant (CMS) from HESCs treated with E2+etonogestrel or E2+MPA under hypoxia induced HEEC apoptosis (Guzeloglu-Kayisli et al., 2014). Moreover, mass spectrometric analysis of this CMS revealed increased levels of neuronal pentraxin-1 (NPTX1), an apoptotic inducer (Clayton et al., 2012; Al Rahim et al., 2013; Thatipamula and Hossain, 2014). As expected, cleaved caspase-3 and NPTX1 immunoreactivity were significantly higher in the endometria from pLARC-treated women. Transcriptomic analysis of these cultures revealed inhibition of AKT signaling and mitochondrial dysfunction in HEECs incubated with HESC-derived CMS. We also found that incubation of HEECs with recombinant NPTX1 ± H2O2 increases both HEEC apoptosis and cytosolic cytochrome c levels. Therefore, in women, pLARC-enhanced NPTX1 secretion and ROS generation in HESCs would impair HEEC survival resulting in loss of vascular integrity leading to AUB (Fig. 4).
The endometrium of pLARC users contains excess neutrophils and NK cells (Hickey et al., 2006a). We observed that Mirena contraception is associated with increased endometrial IL-8 immunoreactivity (Lockwood et al., 2004a). In addition to acting as a well-documented potent chemoattractant for neutrophils, IL-8 also recruits NK cells (Loetscher et al., 1996). Use of the discontinued pLARC, Norplant, was also accompanied by elevated expression by HESCs of the neutrophil activating chemokine, ENA-78 (Chegini et al., 2007). Neutrophils express elastase and MMP-9, and NK cells express several MMPs that exhibit separate and common ECM-degrading specificities (Cundall et al., 2003; Anacker et al., 2011). The combined effects of these molecular changes help account for pLARC-associated AUB (Fig. 4).
Since the abnormal endometrial vasculature observed in pLARC users is characterized by thin-walled hyperdilated fragile microvessels prone to bleeding, we determined whether these vessels are associated with deficient pericytes and vascular smooth muscle cells (VSMCs) support (Kayisli et al., 2015). Double-immunostaining for the presence of proliferating cell nuclear antigen (PCNA) and α-smooth muscle actin (α-SMA) was used to evaluate VSMC differentiation and proliferation respectively in endometria obtained from women before and after pLARC administration. These endometrial vessels displayed significantly reduced α-SMA immunoreactivity and fewer PCNA positive nuclei among α-SMA positive cells. Microarray analysis of primary cultures of human VSMCs treated with either MPA or etonogestrel demonstrated multiple pLARC-regulated genes including decreased CCL2. Of relevance to pLARC induced AUB, while both MPA and etonogestrel reduced VSMC proliferation and migration, recombinant CCL2 reversed this progestin-mediated inhibition. Thus, pLARC appears to inhibit endometrial pericyte and VSCM numbers by inhibiting CCL2 (Fig. 4).
The FK506-binding proteins (FKBP5) belong to a family of immunophilins that mediate actions of specific immunosuppressive drugs. Two members, FKBP51 and FKBP52, share 70% similarity and contain an active peptidyl-prolyl-isomerase domain as well as a tetracopeptide repeat domain that binds to the C-terminus of Hsp90 (Erlejman et al., 2014; Mustafi et al., 2014). This binding enables them to act as co-chaperones with Hsp90 in steroid receptor complexes. While FKBP52 positively regulates the PR, glucocorticoid receptor (GR) and androgen receptor, FKBP51 is a negative steroid receptor regulator (Riggs et al., 2004; Cioffi et al., 2011; Storer et al., 2011; Sanchez, 2012). Over-expression of FKBP51 and PR in human HepG2 cells, decreases progestin responsiveness, suggesting the existence of a negative feedback loop (Hubler et al., 2003). The observation that pLARC treatment does not decrease PR expression in HESCs led us to hypothesize that such treatment increases FKBP51 levels in HESCs, thereby setting into motion a negative feedback loop that impairs the responsiveness of crucial genes to progestin. We recently tested this hypothesis by performing FKBP51 immunohistochemistry on paraffin sections of endometria from women before (pre-) and after three months (post-) Depo MPA (DMPA) administration. In immunostained sections, FKBP51 HSCOREs were significantly higher in endometrial stromal and glandular cells from post-DMPA versus pre-DMPA (Guzeloglu-Kayisli et al., 2015a). To complement this in situ finding in human endometrium, we performed immunostaining on endometrial sections from ovariectomized guinea pigs treated with estradiol or estradiol+MPA for 21 days. In the guinea pigs, estradiol +MPA significantly increased FKBP51 immunoreactivity in endometrial stromal and glandular cells versus estradiol-treatment. Moreover, ingenuity pathway analysis of microarray results from HESC cultures treated with MPA or etonogestrel identified GR activation as an upstream regulator of both MPA and etonogestrel-specific differentially regulated genes, which includes significant enhancement of FKBP51 (Guzeloglu-Kayisli et al., 2015a). Moreover, compared with estradiol, estradiol+MPA treatment significantly inhibited IL-1β mRNA levels in control vector transfected HESCs, whereas MPA failed to inhibit IL-1β mRNA levels in HESCs overexpressing FKBP51. Furthermore, we found that in the presence of MPA, FKBP51 overexpression exacerbates thrombin-induced IL-1β mRNA levels in HESCs (Guzeloglu-Kayisli et al., 2015a). Consequently, chronically increased endometrial FKBP51 expression in pLARC users due to GR signaling activation could contribute to AUB by inducing a negative feedback loop that inhibits PR and GR-mediated transcription. The resultant PR and/or GR-mediated functional withdrawal may contribute to associated endometrial inflammation, aberrant angiogenesis, and bleeding.
Conclusions
Perivascular decidualized HESCs promote endometrial hemostasis during placentation through progestational regulation of hemostatic (TF and PAI-1), anti-fibrinolytic and anti-protease activity, yet paradoxically facilitate menstruation following, progesterone withdrawal via elaboration of fibrinolytic, proteolytic, and vasoconstricting proteins in the setting of reduced TF and PAI-1 expression. Early in pregnancy, the pathological occurrence of decidual hemorrhage is linked to excess local thrombin generation, which contributes to adverse pregnancy outcomes including subsequent pre-eclampsia through multiple biochemical mechanisms including elaboration of sFlt-1 and MMPs, while modifying chemokine expression to enhance numbers of activated macrophages, while inhibiting dNK cell migration. The combined effects of these actions impede spiral artery transformation. These thrombin-induced effects on MMP and chemokine expression are mirrored by TNF-α and to lesser extent by IL-1β, linking inflammatory conditions to pre-eclampsia, and to other adverse pregnancy outcomes. Later in pregnancy, the excess production of these same mediators to cause PTB. Thus, decidual hemorrhage (abruption) leads to excess thrombin generation, which enhances decidual cell MMP and neutrophil infiltration and down-regulates PR expression to preferentially promote PPROM. Similarly, chorioamnionitis-associated IL-1β and to a lesser extent TNF-α, also induce MMPs, inhibit decidual PR expression and also increase COX-2 and PG production to promote PTB due to PPROM and/or preterm labor.
Finally, AUB associated with pLARC use is triggered by unexpected elevation in TF expression at specific bleeding sites accompanied by excess thrombin generation. This increased TF expression is accompanied by reduced endometrial blood flow that promotes hypoxia and ROS-driven aberrant angiogenesis and inflammation as well as progestin induced impairment in pericyte and VSMC envelopment of superficial endometrial vessels. Hypoxia elicits the elaboration of endothelial cell apoptotic inducers secreted by HESCs. The resultant hyper-dilated, thinned wall fragile vessels with disrupted endothelia are the source of AUB leading to high rates of discontinuation of these otherwise safe and effective contraceptives.
Future directions
Worldwide, the PTB rate is 5–18%, accounting for over 15 million births per year (Romero et al., 2014). While the past decade has witnessed an 11% decline in US PTB rates to 11.4% (Schoen et al., 2015), this condition remains the leading cause of perinatal morbidity and mortality in the USA, as well as a major antecedent of chronic lung disease, and neurodevelopmental disorders, accounting for $26 billion/year in health care costs (Rubens et al., 2014). Despite significant research efforts, there has been only modest progress in elucidating underlying mechanisms. Studies focusing on identification of biomarkers are needed for early diagnosis of pregnancy complications leading to PTB. Similarly, little progress has been made in reducing the rate of pLARC-induced AUB. Significant reduction in the rates of PTB or pLARC-induced AUB requires further studies that focus on developing therapeutic agents that block molecules, receptors and/or associated signaling pathways mediating PTB or pLARC-induced AUB. Progress in both areas requires integration of observations made in women with those obtained from relevant animal studies. While the potential contribution of specific transgenic mice is unquestioned, as previously demonstrated by our laboratory (Krikun et al., 2010a, 2012; Kayisli et al., 2015), the guinea pig is a particular appropriate model with which to evaluate reproductive system and pregnancy complications. Thus, like human endometrium, the guinea pig endometrium displays closely related human features such as spontaneous estrus cycling and hemochorial placentation (Krikun et al., 2011).
Authors' roles
F.S., O.G.-K., S.A., U.A.K., and C.J.L. contributed equally to manuscript writing and approved the final version.
Funding
This study was supported by NIH/NICHD 2 RO1 HD 033937 and March of Dimes Foundation Ohio Prematurity Collaborative Grant to C.J.L.
Conflict of interest
None declared.
References
- Affandi B. An integrated analysis of vaginal bleeding patterns in clinical trials of Implanon. Contraception 1998;58:99S–107S. [DOI] [PubMed] [Google Scholar]
- Ahamed J, Ruf W. Protease-activated receptor 2-dependent phosphorylation of the tissue factor cytoplasmic domain. J Biol Chem 2004;279:23038–23044. [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Pepe GJ. Steroid hormone regulation of angiogenesis in the primate endometrium. Front Biosci 2003;8:d416–d429. [DOI] [PubMed] [Google Scholar]
- Al Rahim M, Thatipamula S, Hossain MA. Critical role of neuronal pentraxin 1 in mitochondria-mediated hypoxic-ischemic neuronal injury. Neurobiol Dis 2013;50:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 2004;5:266–271. [DOI] [PubMed] [Google Scholar]
- Anacker J, Segerer SE, Hagemann C, Feix S, Kapp M, Bausch R, Kammerer U. Human decidua and invasive trophoblasts are rich sources of nearly all human matrix metalloproteinases. Mol Hum Reprod 2011;17:637–652. [DOI] [PubMed] [Google Scholar]
- Ananth CV, Peltier MR, Chavez MR, Kirby RS, Getahun D, Vintzileos AM. Recurrence of ischemic placental disease. Obstet Gynecol 2007;110:128–133. [DOI] [PubMed] [Google Scholar]
- Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol 2006;195:803–808. [DOI] [PubMed] [Google Scholar]
- Arcuri F, Toti P, Buchwalder L, Casciaro A, Cintorino M, Schatz F, Rybalov B, Lockwood CJ. Mechanisms of leukocyte accumulation and activation in chorioamnionitis: interleukin 1 beta and tumor necrosis factor alpha enhance colony stimulating factor 2 expression in term decidua. Reprod Sci 2009;16:453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkar AA, Croy BA. Functions of uterine natural killer cells are mediated by interferon gamma production during murine pregnancy. Semin Immunol 2001;13:235–241. [DOI] [PubMed] [Google Scholar]
- Aznaurova YB, Zhumataev MB, Roberts TK, Aliper AM, Zhavoronkov AA. Molecular aspects of development and regulation of endometriosis. Reprod Biol Endocrinol 2014;12:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach RR. Initiation of coagulation by tissue factor. CRC Crit Rev Biochem 1988;23:339–368. [DOI] [PubMed] [Google Scholar]
- Basar M, Yen CF, Buchwalder LF, Murk W, Huang SJ, Godlewski K, Kocamaz E, Arda O, Schatz F, Lockwood CJ et al. . Preeclampsia-related increase of interleukin-11 expression in human decidual cells. Reproduction 2010;140:605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher N, Hein M, Uldbjerg N, Danielsen CC. Balance between matrix metalloproteinases (MMP) and tissue inhibitors of metalloproteinases (TIMP) in the cervical mucus plug estimated by determination of free non-complexed TIMP. Reprod Biol Endocrinol 2008;6:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belting M, Dorrell MI, Sandgren S, Aguilar E, Ahamed J, Dorfleutner A, Carmeliet P, Mueller BM, Friedlander M, Ruf W. Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nat Med 2004;10:502–509. [DOI] [PubMed] [Google Scholar]
- Bergin DA, Reeves EP, Meleady P, Henry M, McElvaney OJ, Carroll TP, Condron C, Chotirmall SH, Clynes M, O'Neill SJ et al. . alpha-1 Antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J Clin Invest 2010;120:4236–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinski MJ, Thorne JG, Oh MJ, Leonard S, Murrant C, Tayade C, Croy BA. Uterine NK cells in murine pregnancy. Reprod Biomed Online 2008;16:218–226. [DOI] [PubMed] [Google Scholar]
- Birgisson NE, Zhao Q, Secura GM, Madden T, Peipert JF. Preventing unintended pregnancy: the contraceptive CHOICE project in review. J Womens Health (Larchmt) 2015;24:349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boij R, Mjosberg J, Svensson-Arvelund J, Hjorth M, Berg G, Matthiesen L, Jenmalm MC, Ernerudh J. Regulatory T-cell subpopulations in severe or early-onset preeclampsia. Am J Reprod Immunol 2015;74:368–378. [DOI] [PubMed] [Google Scholar]
- Bonagura TW, Aberdeen GW, Babischkin JS, Koos RD, Pepe GJ, Albrecht ED. Divergent regulation of angiopoietin-1 and -2, Tie-2, and thrombospondin-1 expression by estrogen in the baboon endometrium. Mol Reprod Dev 2010;77:430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdel N, Alves J, Pickering G, Ramilo I, Roman H, Canis M. Systematic review of endometriosis pain assessment: how to choose a scale? Hum Reprod Update 2015;21:136–152. [DOI] [PubMed] [Google Scholar]
- Bozza M, Bliss JL, Dorner AJ, Trepicchio WL. Interleukin-11 modulates Th1/Th2 cytokine production from activated CD4+ T cells. J Interferon Cytokine Res 2001;21:21–30. [DOI] [PubMed] [Google Scholar]
- Brigstock DR. Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61). Angiogenesis 2002;5:153–165. [DOI] [PubMed] [Google Scholar]
- Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol 2002;187:1416–1423. [DOI] [PubMed] [Google Scholar]
- Brosens JJ, Parker MG, McIndoe A, Pijnenborg R, Brosens IA. A role for menstruation in preconditioning the uterus for successful pregnancy. Am J Obstet Gynecol 2009;200:615 e611–616. [DOI] [PubMed] [Google Scholar]
- Bry K, Hallman M. Prostaglandins, inflammation, and preterm labor. J Perinatol 1989;9:60–65. [PubMed] [Google Scholar]
- Bulun SE. Endometriosis. N Engl J Med 2009;360:268–279. [DOI] [PubMed] [Google Scholar]
- Cakmak H, Schatz F, Huang ST, Buchwalder L, Rahman M, Arici A, Lockwood CJ. Progestin suppresses thrombin- and interleukin-1beta-induced interleukin-11 production in term decidual cells: implications for preterm delivery. J Clin Endocrinol Metab 2005;90:5279–5286. [DOI] [PubMed] [Google Scholar]
- Cakmak H, Guzeloglu-Kayisli O, Kayisli UA, Arici A. Immune-endocrine interactions in endometriosis. Front Biosci (Elite Ed) 2009;1:429–443. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta 2000;21 Suppl A:S25–S30. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van Vlaenderen I, Demunck H, Kasper M, Breier G, Evrard P et al. . Role of tissue factor in embryonic blood vessel development. Nature 1996;383:73–75. [DOI] [PubMed] [Google Scholar]
- Castagnola E, Dufour C. Role of G-CSF GM-CSF in the management of infections in preterm newborns: an update. Early Hum Dev 2014;90 Suppl 2:S15–S17. [DOI] [PubMed] [Google Scholar]
- Chaiworapongsa T, Espinoza J, Yoshimatsu J, Kim YM, Bujold E, Edwin S, Yoon BH, Romero R. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2002;11:368–373. [DOI] [PubMed] [Google Scholar]
- Chaovisitsaree S, Piyamongkol W, Pongsatha S, Morakote N, Noium S, Soonthornlimsiri N. One year study of Implanon on the adverse events and discontinuation. J Med Assoc Thai 2005;88:314–317. [PubMed] [Google Scholar]
- Chegini N, Luo X, Pan Q, Rhoton-Vlasak A, Archer DF. Endometrial expression of epithelial neutrophil-activating peptide-78 during the menstrual cycle or in progestin-only contraceptive users with breakthrough bleeding and the influence of doxycycline therapy. Hum Reprod 2007;22:427–433. [DOI] [PubMed] [Google Scholar]
- Chen CP, Piao L, Chen X, Yu J, Masch R, Schatz F, Lockwood CJ, Huang SJ. Expression of interferon gamma by decidual cells and natural killer cells at the human implantation site: implications for preeclampsia, spontaneous abortion, and intrauterine growth restriction. Reprod Sci 2015;22:1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, Marangos P, Mak I, McVey J, Barker F, White J, Brosens JJ. Interferon-gamma modulates prolactin and tissue factor expression in differentiating human endometrial stromal cells. Endocrinology 2001;142:3142–3151. [DOI] [PubMed] [Google Scholar]
- Church HJ, Vicovac LM, Williams JD, Hey NA, Aplin JD. Laminins 2 and 4 are expressed by human decidual cells. Lab Invest 1996;74:21–32. [PubMed] [Google Scholar]
- Cioffi DL, Hubler TR, Scammell JG. Organization and function of the FKBP52 and FKBP51 genes. Curr Opin Pharmacol 2011;11:308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton KB, Podlesniy P, Figueiro-Silva J, Lopez-Domenech G, Benitez L, Enguita M, Abad MA, Soriano E, Trullas R. NP1 regulates neuronal activity-dependent accumulation of BAX in mitochondria and mitochondrial dynamics. J Neurosci 2012;32:1453–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Meisser A, Bischof P. Metalloproteinases and human placental invasiveness. Placenta 2006;27:783–793. [DOI] [PubMed] [Google Scholar]
- Connolly A, Pietri G, Yu J, Humphreys S. Association between long-acting reversible contraceptive use, teenage pregnancy, and abortion rates in England. Int J Womens Health 2014;6:961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contrino J, Hair G, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nat Med 1996;2:209–215. [DOI] [PubMed] [Google Scholar]
- Cork BA, Tuckerman EM, Li TC, Laird SM. Expression of interleukin (IL)-11 receptor by the human endometrium in vivo and effects of IL-11, IL-6 and LIF on the production of MMP and cytokines by human endometrial cells in vitro. Mol Hum Reprod 2002;8:841–848. [DOI] [PubMed] [Google Scholar]
- Coudyzer P, Lemoine P, Jordan BF, Gallez B, Galant C, Nisolle M, Courtoy PJ, Henriet P, Marbaix E. Hypoxia is not required for human endometrial breakdown or repair in a xenograft model of menstruation. FASEB J 2013;27:3711–3719. [DOI] [PubMed] [Google Scholar]
- Critchley HO, Saunders PT. Hormone receptor dynamics in a receptive human endometrium. Reprod Sci 2009;16:191–199. [DOI] [PubMed] [Google Scholar]
- Cundall M, Sun Y, Miranda C, Trudeau JB, Barnes S, Wenzel SE. Neutrophil-derived matrix metalloproteinase-9 is increased in severe asthma and poorly inhibited by glucocorticoids. J Allergy Clin Immunol 2003;112:1064–1071. [DOI] [PubMed] [Google Scholar]
- Curti A, Ratta M, Corinti S, Girolomoni G, Ricci F, Tazzari P, Siena M, Grande A, Fogli M, Tura S et al. . Interleukin-11 induces Th2 polarization of human CD4(+) T cells. Blood 2001;97:2758–2763. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Fisher SJ. Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr Opin Cell Biol 1998;10:660–666. [DOI] [PubMed] [Google Scholar]
- Dimberg A. Chemokines in angiogenesis. Curr Top Microbiol Immunol 2010;341:59–80. [DOI] [PubMed] [Google Scholar]
- Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- Draxler DF, Medcalf RL. The fibrinolytic system-more than fibrinolysis? Transfus Med Rev 2015;29:102–109. [DOI] [PubMed] [Google Scholar]
- Du MR, Guo PF, Piao HL, Wang SC, Sun C, Jin LP, Tao Y, Li YH, Zhang D, Zhu R et al. . Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal-fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells. J Immunol 2014;192:1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duanmu J, Cheng J, Xu J, Booth CJ, Hu Z. Effective treatment of chemoresistant breast cancer in vitro and in vivo by a factor VII-targeted photodynamic therapy. Br J Cancer 2011;104:1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovitz MA, Baron J, Phillippe M. The role of thrombin in preterm parturition. Am J Obstet Gynecol 2001;185:1059–1063. [DOI] [PubMed] [Google Scholar]
- Equils O, Singh S, Karaburun S, Lu D, Thamotharan M, Devaskar SU. Intra-uterine growth restriction downregulates the hepatic toll like receptor-4 expression and function. Clin Dev Immunol 2005;12:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlejman AG, Lagadari M, Harris DC, Cox MB, Galigniana MD. Molecular chaperone activity and biological regulatory actions of the TPR-domain immunophilins FKBP51 and FKBP52. Curr Protein Pept Sci 2014;15:205–215. [DOI] [PubMed] [Google Scholar]
- Eroglu Z, Stein CA, Pal SK. Targeting angiopoietin-2 signaling in cancer therapy. Expert Opin Investig Drugs 2013;22:813–825. [DOI] [PubMed] [Google Scholar]
- Faas MM, Spaans F, De Vos P. Monocytes and macrophages in pregnancy and pre-eclampsia. Front Immunol 2014;5:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Rai A, Kambham N, Sung JF, Singh N, Petitt M, Dhal S, Agrawal R, Sutton RE, Druzin ML et al. . Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J Clin Invest 2014;124:4941–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Herrera H, Garcia-Lopez G, Diaz NF, Molina-Hernandez A, Osorio-Caballero M, Soriano-Becerril D, Zaga-Clavellina V. An experimental mixed bacterial infection induced differential secretion of proinflammatory cytokines (IL-1beta, TNFalpha) and proMMP-9 in human fetal membranes. Placenta 2012;33:271–277. [DOI] [PubMed] [Google Scholar]
- Fortunato SJ, Menon R, Lombardi SJ. Stromelysins in placental membranes and amniotic fluid with premature rupture of membranes. Obstet Gynecol 1999;94:435–440. [DOI] [PubMed] [Google Scholar]
- Fu Q, Cheng J, Gao Y, Zhang Y, Chen X, Xie J. Protease-activated receptor 4: a critical participator in inflammatory response. Inflammation 2015;38:886–895. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Sako K, Noda K, Zhang J, Minami M, Mochizuki N. Angiopoietin-1/Tie2 receptor signaling in vascular quiescence and angiogenesis. Histol Histopathol 2010;25:387–396. [DOI] [PubMed] [Google Scholar]
- Gales D, Clark C, Manne U, Samuel T. The chemokine CXCL8 in carcinogenesis and drug response. ISRN Oncol 2013;2013:859154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperini S, Marchi M, Calzetti F, Laudanna C, Vicentini L, Olsen H, Murphy M, Liao F, Farber J, Cassatella MA. Gene expression and production of the monokine induced by IFN-gamma (MIG), IFN-inducible T cell alpha chemoattractant (I-TAC), and IFN-gamma-inducible protein-10 (IP-10) chemokines by human neutrophils. J Immunol 1999;162:4928–4937. [PubMed] [Google Scholar]
- Gerald D, Chintharlapalli S, Augustin HG, Benjamin LE. Angiopoietin-2: an attractive target for improved antiangiogenic tumor therapy. Cancer Res 2013;73:1649–1657. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Kao LC. Endometriosis. Lancet 2004;364:1789–1799. [DOI] [PubMed] [Google Scholar]
- Gordon MS, McCaskill-Stevens WJ, Battiato LA, Loewy J, Loesch D, Breeden E, Hoffman R, Beach KJ, Kuca B, Kaye J et al. . A phase I trial of recombinant human interleukin-11 (neumega rhIL-11 growth factor) in women with breast cancer receiving chemotherapy. Blood 1996;87:3615–3624. [PubMed] [Google Scholar]
- Greenwood JD, Minhas K, di Santo JP, Makita M, Kiso Y, Croy BA. Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta 2000;21:693–702. [DOI] [PubMed] [Google Scholar]
- Grimaldi G, Christian M, Quenby S, Brosens JJ. Expression of epigenetic effectors in decidualizing human endometrial stromal cells. Mol Hum Reprod 2012;18:451–458. [DOI] [PubMed] [Google Scholar]
- Guenther S, Vrekoussis T, Heublein S, Bayer B, Anz D, Knabl J, Navrozoglou I, Dian D, Friese K, Makrigiannakis A et al. . Decidual macrophages are significantly increased in spontaneous miscarriages and over-express FasL: a potential role for macrophages in trophoblast apoptosis. Int J Mol Sci 2012;13:9069–9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzeloglu-Kayisli O, Basar M, Shapiro JP, Semerci N, Huang JS, Schatz F, Lockwood CJ, Kayisli UA. Long-acting progestin-only contraceptives enhance human endometrial stromal cell expressed neuronal pentraxin-1 and reactive oxygen species to promote endothelial cell apoptosis. J Clin Endocrinol Metab 2014;99:E1957–E1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzeloglu-Kayisli O, Kayisli UA, Basar M, Semerci N, Schatz F, Lockwood CJ. Enhanced expression of FKBP51 in endometrium of women using long-acting progestin only contraceptives: implications for functional progesterone and glucocorticoid withdrawal. PLoS One 2015a;10:e0137855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzeloglu-Kayisli O, Kayisli UA, Semerci N, Basar M, Buchwalder LF, Buhimschi CS, Buhimschi IA, Arcuri F, Larsen K, Huang JS et al. . Mechanisms of chorioamnionitis-associated preterm birth: interleukin-1beta inhibits progesterone receptor expression in decidual cells. J Pathol 2015b;237:423–434. [DOI] [PubMed] [Google Scholar]
- Hafeez NA, Fouda Mel T, Abdel Gawad ER, Assar T, Mansour AI. The role of regulatory T cells in preeclampsia. Egypt J Immunol 2014;21:45–55. [PubMed] [Google Scholar]
- Han SJ, O'Malley BW. The dynamics of nuclear receptors and nuclear receptor coregulators in the pathogenesis of endometriosis. Hum Reprod Update 2014;20:467–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Wald O, Goldman-Wohl D, Prus D, Markel G, Gazit R, Katz G, Haimov-Kochman R, Fujii N, Yagel S et al. . CXCL12 expression by invasive trophoblasts induces the specific migration of CD16- human natural killer cells. Blood 2003;102:1569–1577. [DOI] [PubMed] [Google Scholar]
- Hansen PJ, Dobbs KB, Denicol AC. Programming of the preimplantation embryo by the embryokine colony stimulating factor 2. Anim Reprod Sci 2014;149:59–66. [DOI] [PubMed] [Google Scholar]
- Harger JH, Hsing AW, Tuomala RE, Gibbs RS, Mead PB, Eschenbach DA, Knox GE, Polk BF. Risk factors for preterm premature rupture of fetal membranes: a multicenter case-control study. Am J Obstet Gynecol 1990;163:130–137. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Shibasaki F. Hypoxia-inducible factor as an angiogenic master switch. Front Pediatr 2015;3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriet P, Gaide Chevronnay HP, Marbaix E. The endocrine and paracrine control of menstruation. Mol Cell Endocrinol 2012;358:197–207. [DOI] [PubMed] [Google Scholar]
- Hickey M, Crewe J, Mahoney LA, Doherty DA, Fraser IS, Salamonsen LA. Mechanisms of irregular bleeding with hormone therapy: the role of matrix metalloproteinases and their tissue inhibitors. J Clin Endocrinol Metab 2006a;91:3189–3198. [DOI] [PubMed] [Google Scholar]
- Hickey M, Krikun G, Kodaman P, Schatz F, Carati C, Lockwood CJ. Long-term progestin-only contraceptives result in reduced endometrial blood flow and oxidative stress. J Clin Endocrinol Metab 2006b;91:3633–3638. [DOI] [PubMed] [Google Scholar]
- Hirota Y, Osuga Y, Hirata T, Yoshino O, Koga K, Harada M, Morimoto C, Nose E, Yano T, Tsutsumi O et al. . Possible involvement of thrombin/protease-activated receptor 1 system in the pathogenesis of endometriosis. J Clin Endocrinol Metab 2005;90:3673–3679. [DOI] [PubMed] [Google Scholar]
- Hu Z, Garen A. Intratumoral injection of adenoviral vectors encoding tumor-targeted immunoconjugates for cancer immunotherapy. Proc Natl Acad Sci USA 2000;97:9221–9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Garen A. Targeting tissue factor on tumor vascular endothelial cells and tumor cells for immunotherapy in mouse models of prostatic cancer. Proc Natl Acad Sci USA 2001;98:12180–12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Sun Y, Garen A. Targeting tumor vasculature endothelial cells and tumor cells for immunotherapy of human melanoma in a mouse xenograft model. Proc Natl Acad Sci USA 1999;96:8161–8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SJ, Schatz F, Masch R, Rahman M, Buchwalder L, Niven-Fairchild T, Tang C, Abrahams VM, Krikun G, Lockwood CJ. Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Reprod Immunol 2006;72:60–73. [DOI] [PubMed] [Google Scholar]
- Huang SJ, Zenclussen AC, Chen CP, Basar M, Yang H, Arcuri F, Li M, Kocamaz E, Buchwalder L, Rahman M et al. . The implication of aberrant GM-CSF expression in decidual cells in the pathogenesis of preeclampsia. Am J Pathol 2010;177:2472–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology 2003;144:2380–2387. [DOI] [PubMed] [Google Scholar]
- Huppertz B. Maternal and fetal factors and placentation: implications for pre-eclampsia. Pregnancy Hypertens 2014a;4:244. [DOI] [PubMed] [Google Scholar]
- Huppertz B. Oxygenation of the placenta and its role in pre-eclampsia. Pregnancy Hypertens 2014b;4:244–245. [DOI] [PubMed] [Google Scholar]
- Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell 1993;4:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Nagase H. Preferential inactivation of tissue inhibitor of metalloproteinases-1 that is bound to the precursor of matrix metalloproteinase 9 (progelatinase B) by human neutrophil elastase. J Biol Chem 1995;270:16518–16521. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 2003;130:2779–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahashi M, Muragaki Y, Ooshima A, Yamoto M, Nakano R. Alterations in distribution and composition of the extracellular matrix during decidualization of the human endometrium. J Reprod Fertil 1996;108:147–155. [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev 2006;27:17–46. [DOI] [PubMed] [Google Scholar]
- Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol 2005;175:3463–3468. [DOI] [PubMed] [Google Scholar]
- Kaitu'u TJ, Shen J, Zhang J, Morison NB, Salamonsen LA. Matrix metalloproteinases in endometrial breakdown and repair: functional significance in a mouse model. Biol Reprod 2005;73:672–680. [DOI] [PubMed] [Google Scholar]
- Kaunitz AM. Injectable contraception. New and existing options. Obstet Gynecol Clin North Am 2000;27:741–780. [DOI] [PubMed] [Google Scholar]
- Kayisli UA, Berkkanoglu M, Zhang L, Kizilay G, Arici A. The broad-spectrum chemokine inhibitor NR58-3.14.3 suppresses the implantation and survival of human endometrial implants in the nude mice endometriosis model. Reprod Sci 2007;14:825–835. [DOI] [PubMed] [Google Scholar]
- Kayisli UA, Basar M, Guzeloglu-Kayisli O, Semerci N, Atkinson HC, Shapiro J, Summerfield T, Huang SJ, Prelle K, Schatz F et al. . Long-acting progestin-only contraceptives impair endometrial vasculature by inhibiting uterine vascular smooth muscle cell survival. Proc Natl Acad Sci USA 2015;112:5153–5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh GY. Orchestral actions of angiopoietin-1 in vascular regeneration. Trends Mol Med 2013;19:31–39. [DOI] [PubMed] [Google Scholar]
- Kopcow HD, Karumanchi SA. Angiogenic factors and natural killer (NK) cells in the pathogenesis of preeclampsia. J Reprod Immunol 2007;76:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikun G, Lockwood CJ. Steroid hormones, endometrial gene regulation and the Sp1 family of proteins. J Soc Gynecol Investig 2002;9:329–334. [PubMed] [Google Scholar]
- Krikun G, Schatz F, Finlay T, Kadner S, Mesia A, Gerrets R, Lockwood CJ. Expression of angiopoietin-2 by human endometrial endothelial cells: regulation by hypoxia and inflammation. Biochem Biophys Res Commun 2000a;275:159–163. [DOI] [PubMed] [Google Scholar]
- Krikun G, Schatz F, Mackman N, Guller S, Demopoulos R, Lockwood CJ. Regulation of tissue factor gene expression in human endometrium by transcription factors Sp1 and Sp3. Mol Endocrinol 2000b;14:393–400. [DOI] [PubMed] [Google Scholar]
- Krikun G, Critchley H, Schatz F, Wan L, Caze R, Baergen RN, Lockwood CJ. Abnormal uterine bleeding during progestin-only contraception may result from free radical-induced alterations in angiopoietin expression. Am J Pathol 2002;161:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikun G, Sakkas D, Schatz F, Buchwalder L, Hylton D, Tang C, Lockwood CJ. Endometrial angiopoietin expression and modulation by thrombin and steroid hormones: a mechanism for abnormal angiogenesis following long-term progestin-only contraception. Am J Pathol 2004;164:2101–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikun G, Schatz F, Taylor H, Lockwood CJ. Endometriosis and tissue factor. Ann N Y Acad Sci 2008;1127:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikun G, Buhimschi IA, Hickey M, Schatz F, Buchwalder L, Lockwood CJ. Long-term progestin contraceptives (LTPOC) induce aberrant angiogenesis, oxidative stress and apoptosis in the guinea pig uterus: a model for abnormal uterine bleeding in humans. J Angiogenes Res 2010a;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikun G, Hu Z, Osteen K, Bruner-Tran KL, Schatz F, Taylor HS, Toti P, Arcuri F, Konigsberg W, Garen A et al. . The immunoconjugate ‘icon’ targets aberrantly expressed endothelial tissue factor causing regression of endometriosis. Am J Pathol 2010b;176:1050–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikun G, Booth CJ, Buchwalder L, Caze R, Rahman M, Schatz F, Buhimschi I, Lockwood C. Long-term progestin-only contraception in humans versus animal models. Ann N Y Acad Sci 2011;1221:119–123. [DOI] [PubMed] [Google Scholar]
- Krikun G, Booth CJ, Buchwalder L, Schatz F, Osol G, Mandala M, Lockwood CJ. Effects of etonogestrel treatment in the reproductive organs and uterine arteries of nonoophorectomized guinea pigs. Reprod Sci 2012;19:400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Schatz F, Moore RM, Mercer BM, Rangaswamy N, Mansour JM, Lockwood CJ, Moore JJ. The effects of thrombin and cytokines upon the biomechanics and remodeling of isolated amnion membrane, in vitro. Placenta 2011;32:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Moore RM, Nash A, Springel E, Mercer BM, Philipson E, Mansour JM, Moore JJ. Decidual GM-CSF is a critical common intermediate necessary for thrombin and TNF induced in-vitro fetal membrane weakening. Placenta 2014;35:1049–1056. [DOI] [PubMed] [Google Scholar]
- Lash GE, Robson SC, Bulmer JN. Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta 2010;31 Suppl:S87–S92. [DOI] [PubMed] [Google Scholar]
- Lau TM, Affandi B, Rogers PA. The effects of levonorgestrel implants on vascular endothelial growth factor expression in the endometrium. Mol Hum Reprod 1999;5:57–63. [DOI] [PubMed] [Google Scholar]
- Leber A, Teles A, Zenclussen AC. Regulatory T cells and their role in pregnancy. Am J Reprod Immunol 2010;63:445–459. [DOI] [PubMed] [Google Scholar]
- Leppert U, Eisenreich A. The role of tissue factor isoforms in cancer biology. Int J Cancer 2015;137:497–503. [DOI] [PubMed] [Google Scholar]
- Lijnen HR. Matrix metalloproteinases and cellular fibrinolytic activity. Biochemistry (Mosc) 2002;67:92–98. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ. Regulation of plasminogen activator inhibitor 1 expression by interaction of epidermal growth factor with progestin during decidualization of human endometrial stromal cells. Am J Obstet Gynecol 2001;184:798–804; discussion 804–805. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Senyei AE, Dische MR, Casal D, Shah KD, Thung SN, Jones L, Deligdisch L, Garite TJ. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med 1991;325:669–674. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Nemerson Y, Guller S, Krikun G, Alvarez M, Hausknecht V, Gurpide E, Schatz F. Progestational regulation of human endometrial stromal cell tissue factor expression during decidualization. J Clin Endocrinol Metab 1993a;76:231–236. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Nemerson Y, Krikun G, Hausknecht V, Markiewicz L, Alvarez M, Guller S, Schatz F. Steroid-modulated stromal cell tissue factor expression: a model for the regulation of endometrial hemostasis and menstruation. J Clin Endocrinol Metab 1993b;77:1014–1019. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Ghidini A, Wein R, Lapinski R, Casal D, Berkowitz RL. Increased interleukin-6 concentrations in cervical secretions are associated with preterm delivery. Am J Obstet Gynecol 1994a;171:1097–1102. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Krikun G, Papp C, Aigner S, Nemerson Y, Schatz F. Biological mechanisms underlying RU 486 clinical effects: inhibition of endometrial stromal cell tissue factor content. J Clin Endocrinol Metab 1994b;79:786–790. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Krikun G, Papp C, Aigner S, Schatz F. Biological mechanisms underlying the clinical effects of RU 486: modulation of cultured endometrial stromal cell plasminogen activator and plasminogen activator inhibitor expression. J Clin Endocrinol Metab 1995;80:1100–1105. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Krikun G, Hausknecht VA, Papp C, Schatz F. Matrix metalloproteinase and matrix metalloproteinase inhibitor expression in endometrial stromal cells during progestin-initiated decidualization and menstruation-related progestin withdrawal. Endocrinology 1998;139:4607–4613. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Krikun G, Runic R, Schwartz LB, Mesia AF, Schatz F. Progestin-epidermal growth factor regulation of tissue factor expression during decidualization of human endometrial stromal cells. J Clin Endocrinol Metab 2000;85:297–301. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Krikun G, Koo AB, Kadner S, Schatz F. Differential effects of thrombin and hypoxia on endometrial stromal and glandular epithelial cell vascular endothelial growth factor expression. J Clin Endocrinol Metab 2002;87:4280–4286. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Kumar P, Krikun G, Kadner S, Dubon P, Critchley H, Schatz F. Effects of thrombin, hypoxia, and steroids on interleukin-8 expression in decidualized human endometrial stromal cells: implications for long-term progestin-only contraceptive-induced bleeding. J Clin Endocrinol Metab 2004a;89:1467–1475. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Schatz F, Krikun G. Angiogenic factors and the endometrium following long term progestin only contraception. Histol Histopathol 2004b;19:167–172. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Toti P, Arcuri F, Paidas M, Buchwalder L, Krikun G, Schatz F. Mechanisms of abruption-induced premature rupture of the fetal membranes: thrombin-enhanced interleukin-8 expression in term decidua. Am J Pathol 2005;167:1443–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood CJ, Arcuri F, Toti P, Felice CD, Krikun G, Guller S, Buchwalder LF, Schatz F. Tumor necrosis factor-alpha and interleukin-1beta regulate interleukin-8 expression in third trimester decidual cells: implications for the genesis of chorioamnionitis. Am J Pathol 2006a;169:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood CJ, Matta P, Krikun G, Koopman LA, Masch R, Toti P, Arcuri F, Huang ST, Funai EF, Schatz F. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia. Am J Pathol 2006b;168:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood CJ, Krikun G, Rahman M, Caze R, Buchwalder L, Schatz F. The role of decidualization in regulating endometrial hemostasis during the menstrual cycle, gestation, and in pathological states. Semin Thromb Hemost 2007a;33:111–117. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Toti P, Arcuri F, Norwitz E, Funai EF, Huang ST, Buchwalder LF, Krikun G, Schatz F. Thrombin regulates soluble fms-like tyrosine kinase-1 (sFlt-1) expression in first trimester decidua: implications for preeclampsia. Am J Pathol 2007b;170:1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood CJ, Oner C, Uz YH, Kayisli UA, Huang SJ, Buchwalder LF, Murk W, Funai EF, Schatz F. Matrix metalloproteinase 9 (MMP9) expression in preeclamptic decidua and MMP9 induction by tumor necrosis factor alpha and interleukin 1 beta in human first trimester decidual cells. Biol Reprod 2008;78:1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood CJ, Murk W, Kayisli UA, Buchwalder LF, Huang ST, Funai EF, Krikun G, Schatz F. Progestin and thrombin regulate tissue factor expression in human term decidual cells. J Clin Endocrinol Metab 2009;94:2164–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood CJ, Murk WK, Kayisli UA, Buchwalder LF, Huang SJ, Arcuri F, Li M, Gopinath A, Schatz F. Regulation of interleukin-6 expression in human decidual cells and its potential role in chorioamnionitis. Am J Pathol 2010;177:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood CJ, Kayisli UA, Stocco C, Murk W, Vatandaslar E, Buchwalder LF, Schatz F. Abruption-induced preterm delivery is associated with thrombin-mediated functional progesterone withdrawal in decidual cells. Am J Pathol 2012;181:2138–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood CJ, Huang SJ, Chen CP, Huang Y, Xu J, Faramarzi S, Kayisli O, Kayisli U, Koopman L, Smedts D et al. . Decidual cell regulation of natural killer cell-recruiting chemokines: implications for the pathogenesis and prediction of preeclampsia. Am J Pathol 2013;183:841–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood CJ, Basar M, Kayisli UA, Guzeloglu-Kayisli O, Murk W, Wang J, De Paz N, Shapiro JP, Masch RJ, Semerci N et al. . Interferon-gamma protects first-trimester decidual cells against aberrant matrix metalloproteinases 1, 3, and 9 expression in preeclampsia. Am J Pathol 2014;184:2549–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B. Activation of NK cells by CC chemokines. Chemotaxis, Ca2+ mobilization, and enzyme release. J Immunol 1996;156:322–327. [PubMed] [Google Scholar]
- Lucas ES, Dyer NP, Murakami K, Lee YH, Chan YW, Grimaldi G, Muter J, Brighton PJ, Moore JD, Patel G et al. . Loss of endometrial plasticity in recurrent pregnancy loss. Stem Cells 2015;34:346–356. [DOI] [PubMed] [Google Scholar]
- Lyall F. Priming and remodelling of human placental bed spiral arteries during pregnancy—a review. Placenta 2005;26 Suppl A:S31–S36. [DOI] [PubMed] [Google Scholar]
- Lykke JA, Dideriksen KL, Lidegaard O, Langhoff-Roos J. First-trimester vaginal bleeding and complications later in pregnancy. Obstet Gynecol 2010a;115:935–944. [DOI] [PubMed] [Google Scholar]
- Lykke JA, Langhoff-Roos J, Lockwood CJ, Triche EW, Paidas MJ. Mortality of mothers from cardiovascular and non-cardiovascular causes following pregnancy complications in first delivery. Paediatr Perinat Epidemiol 2010b;24:323–330. [DOI] [PubMed] [Google Scholar]
- Mackenzie AP, Schatz F, Krikun G, Funai EF, Kadner S, Lockwood CJ. Mechanisms of abruption-induced premature rupture of the fetal membranes: thrombin enhanced decidual matrix metalloproteinase-3 (stromelysin-1) expression. Am J Obstet Gynecol 2004;191:1996–2001. [DOI] [PubMed] [Google Scholar]
- Macklon NS, Brosens JJ. The human endometrium as a sensor of embryo quality. Biol Reprod 2014;91:98. [DOI] [PubMed] [Google Scholar]
- Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol 2004;24:1015–1022. [DOI] [PubMed] [Google Scholar]
- Mai KT, Yazdi HM, Perkins DG, Parks W. Development of endometriosis from embryonic duct remnants. Hum Pathol 1998;29:319–322. [DOI] [PubMed] [Google Scholar]
- Male V, Hughes T, McClory S, Colucci F, Caligiuri MA, Moffett A. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J Immunol 2010;185:3913–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck TA, Esplin MS, Biggio J, Bukowski R, Parry S, Zhang H, Huang H, Varner MW, Andrews W, Saade G et al. . The phenotype of spontaneous preterm birth: application of a clinical phenotyping tool. Am J Obstet Gynecol 2015;212:487 e481–487 e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbaix E, Donnez J, Courtoy PJ, Eeckhout Y. Progesterone regulates the activity of collagenase and related gelatinases A and B in human endometrial explants. Proc Natl Acad Sci USA 1992;89:11789–11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh MM, Findlay JK, Salamonsen LA. Endothelin and menstruation. Hum Reprod 1996;11 Suppl 2:83–89. [DOI] [PubMed] [Google Scholar]
- Matias ML, Romao M, Weel IC, Ribeiro VR, Nunes PR, Borges VT, Araujo JP Jr, Peracoli JC, de Oliveira L, Peracoli MT. Endogenous and uric acid-induced activation of NLRP3 inflammasome in pregnant women with preeclampsia. PLoS One 2015;10:e0129095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta P, Lockwood CJ, Schatz F, Krikun G, Rahman M, Buchwalder L, Norwitz ER. Thrombin regulates monocyte chemoattractant protein-1 expression in human first trimester and term decidual cells. Am J Obstet Gynecol 2007;196:268 e261–268. [DOI] [PubMed] [Google Scholar]
- Maybin JA, Critchley HO. Menstrual physiology: implications for endometrial pathology and beyond. Hum Reprod Update 2015;21:748–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maybin JA, Battersby S, Hirani N, Nikitenko LL, Critchley HO, Jabbour HN. The expression and regulation of adrenomedullin in the human endometrium: a candidate for endometrial repair. Endocrinology 2011a;152:2845–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maybin JA, Hirani N, Brown P, Jabbour HN, Critchley HO. The regulation of vascular endothelial growth factor by hypoxia and prostaglandin F(2)alpha during human endometrial repair. J Clin Endocrinol Metab 2011b;96:2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maybin JA, Barcroft J, Thiruchelvam U, Hirani N, Jabbour HN, Critchley HO. The presence and regulation of connective tissue growth factor in the human endometrium. Hum Reprod 2012;27:1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maymon E, Romero R, Pacora P, Gervasi MT, Bianco K, Ghezzi F, Yoon BH. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol 2000;183:914–920. [DOI] [PubMed] [Google Scholar]
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE et al. . Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon K, Karumanchi SA, Stillman IE, Cummings P, Patton D, Easterling T. Does soluble fms-like tyrosine kinase-1 regulate placental invasion? Insight from the invasive placenta. Am J Obstet Gynecol 2014;210:68 e61–64. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454:428–435. [DOI] [PubMed] [Google Scholar]
- Melgert BN, Spaans F, Borghuis T, Klok PA, Groen B, Bolt A, de Vos P, van Pampus MG, Wong TY, van Goor H et al. . Pregnancy and preeclampsia affect monocyte subsets in humans and rats. PLoS One 2012;7:e45229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogami H, Keller PW, Shi H, Word RA. Effect of thrombin on human amnion mesenchymal cells, mouse fetal membranes, and preterm birth. J Biol Chem 2014;289:13295–13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk JM, Leonard S, McBey BA, Croy BA. Induction of murine spiral artery modification by recombinant human interferon-gamma. Placenta 2005;26:835–838. [DOI] [PubMed] [Google Scholar]
- Morgan H, Tumber A, Hill PA. Breast cancer cells induce osteoclast formation by stimulating host IL-11 production and downregulating granulocyte/macrophage colony-stimulating factor. Int J Cancer 2004;109:653–660. [DOI] [PubMed] [Google Scholar]
- Morinaga Y, Fujita N, Ohishi K, Zhang Y, Tsuruo T. Suppression of interleukin-11-mediated bone resorption by cyclooxygenases inhibitors. J Cell Physiol 1998;175:247–254. [DOI] [PubMed] [Google Scholar]
- Mustafi SM, LeMaster DM, Hernandez G. Differential conformational dynamics in the closely homologous FK506-binding domains of FKBP51 and FKBP52. Biochem J 2014;461:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutihir JT, Daru PH. Implanon sub-dermal implants: a 10-month review of acceptability in Jos, North-Central Nigeria. Niger J Clin Pract 2008;11:320–323. [PubMed] [Google Scholar]
- Naeye RL. Maternal age, obstetric complications, and the outcome of pregnancy. Obstet Gynecol 1983;61:210–216. [PubMed] [Google Scholar]
- Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, Momoeda M, Kozuma S, Taketani Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 2004;145:4838–4845. [DOI] [PubMed] [Google Scholar]
- Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem 1999;274:21491–21494. [DOI] [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 2006;69:562–573. [DOI] [PubMed] [Google Scholar]
- Nagayama S, Ohkuchi A, Shirasuna K, Takahashi K, Suzuki H, Hirashima C, Sakata A, Nishimura S, Takahashi M, Matsubara S. The frequency of peripheral blood CD4FoxP3 regulatory T cells in women with pre-eclampsia and those with high-risk factors for pre-eclampsia. Hypertens Pregnancy 2015;34:443–455. [DOI] [PubMed] [Google Scholar]
- Naruse K, Lash GE, Innes BA, Otun HA, Searle RF, Robson SC, Bulmer JN. Localization of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitors for MMPs (TIMPs) in uterine natural killer cells in early human pregnancy. Hum Reprod 2009;24:553–561. [DOI] [PubMed] [Google Scholar]
- North RA, McCowan LM, Dekker GA, Poston L, Chan EH, Stewart AW, Black MA, Taylor RS, Walker JJ, Baker PN et al. . Clinical risk prediction for pre-eclampsia in nulliparous women: development of model in international prospective cohort. BMJ 2011;342:d1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohbuchi H, Nagai K, Yamaguchi M, Ikenoue T, Mori N, Kitamura K, Araki S, Toshimori K. Endothelin-1 and big endothelin-1 increase in human endometrium during menstruation. Am J Obstet Gynecol 1995;173:1483–1490. [DOI] [PubMed] [Google Scholar]
- Okada H, Tsuzuki T, Shindoh H, Nishigaki A, Yasuda K, Kanzaki H. Regulation of decidualization and angiogenesis in the human endometrium: mini review. J Obstet Gynaecol Res 2014;40:1180–1187. [DOI] [PubMed] [Google Scholar]
- Oner C, Schatz F, Kizilay G, Murk W, Buchwalder LF, Kayisli UA, Arici A, Lockwood CJ. Progestin-inflammatory cytokine interactions affect matrix metalloproteinase-1 and -3 expression in term decidual cells: implications for treatment of chorioamnionitis-induced preterm delivery. J Clin Endocrinol Metab 2008;93:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi NM, Tribe RM. Cytokine networks and the regulation of uterine function in pregnancy and parturition. J Neuroendocrinol 2008;20:462–469. [DOI] [PubMed] [Google Scholar]
- Osuga Y, Hirota Y, Yoshino O, Hirata T, Koga K, Taketani Y. Proteinase-activated receptors in the endometrium and endometriosis. Front Biosci (Schol Ed) 2012a;4:1201–1212. [DOI] [PubMed] [Google Scholar]
- Osuga Y, Hirota Y, Yoshino O, Hirata T, Koga K, Taketani Y. Proteinase-activated receptors in the endometrium and endometriosis. Front Biosci (Elite Ed) 2012b;4:755–767. [DOI] [PubMed] [Google Scholar]
- Oyelese Y, Ananth CV. Placental abruption. Obstet Gynecol 2006;108:1005–1016. [DOI] [PubMed] [Google Scholar]
- Parry GC, Erlich JH, Carmeliet P, Luther T, Mackman N. Low levels of tissue factor are compatible with development and hemostasis in mice. J Clin Invest 1998;101:560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update 2015;21:155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff D, Fiedler U, Augustin HG. Emerging roles of the Angiopoietin-Tie and the ephrin-Eph systems as regulators of cell trafficking. J Leukoc Biol 2006;80:719–726. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 2006;27:939–958. [DOI] [PubMed] [Google Scholar]
- Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011;123:2856–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presicce P, Senthamaraikannan P, Alvarez M, Rueda CM, Cappelletti M, Miller LA, Jobe AH, Chougnet CA, Kallapur SG. Neutrophil recruitment and activation in decidua with intra-amniotic IL-1beta in the preterm rhesus macaque. Biol Reprod 2015;92:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritts EA, Ryan IP, Mueller MD, Lebovic DI, Shifren JL, Zaloudek CJ, Korn AP, Darney PD, Taylor RN. Angiogenic effects of norplant contraception on endometrial histology and uterine bleeding. J Clin Endocrinol Metab 2005;90:2142–2147. [DOI] [PubMed] [Google Scholar]
- Quesniaux VF, Clark SC, Turner K, Fagg B. Interleukin-11 stimulates multiple phases of erythropoiesis in vitro. Blood 1992;80:1218–1223. [PubMed] [Google Scholar]
- Quinn KH, Parast MM. Decidual regulatory T cells in placental pathology and pregnancy complications. Am J Reprod Immunol 2013;69:533–538. [DOI] [PubMed] [Google Scholar]
- Rafie S, McIntosh J, Shealy KM, Borgelt LM, Forinash A, Shrader SP, Koepf ER, McClendon KS, Griffin BL, Horlen C et al. . Roles of the pharmacist in the use of safe and highly effective long-acting reversible contraception: an opinion of the women's health practice and research network of the American College of Clinical Pharmacy. Pharmacotherapy 2014;34:991–999. [DOI] [PubMed] [Google Scholar]
- Rahimzadeh M, Norouzian M, Arabpour F, Naderi N. Regulatory T-cells and preeclampsia: an overview of literature. Expert Rev Clin Immunol 2016;12:209–227. [DOI] [PubMed] [Google Scholar]
- Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta 2005;26:563–573. [DOI] [PubMed] [Google Scholar]
- Riggs DL, Cox MB, Cheung-Flynn J, Prapapanich V, Carrigan PE, Smith DF. Functional specificity of co-chaperone interactions with Hsp90 client proteins. Crit Rev Biochem Mol Biol 2004;39:279–295. [DOI] [PubMed] [Google Scholar]
- Rinehart BK, Terrone DA, Isler CM, Barrilleaux PS, Bufkin L, Morrison JC. Pregnancy outcome in women with preterm labor symptoms without cervical change. Am J Obstet Gynecol 2001;184:1004–1007. [DOI] [PubMed] [Google Scholar]
- Robson A, Harris LK, Innes BA, Lash GE, Aljunaidy MM, Aplin JD, Baker PN, Robson SC, Bulmer JN. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J 2012;26:4876–4885. [DOI] [PubMed] [Google Scholar]
- Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. Infection in the pathogenesis of preterm labor. Semin Perinatol 1988;12:262–279. [PubMed] [Google Scholar]
- Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol 2002;7:259–274. [DOI] [PubMed] [Google Scholar]
- Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014;345:760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopa BA, Loganath A, Singh K. The effect of a levonorgestrel-releasing intrauterine system on angiogenic growth factors in the endometrium. Hum Reprod 2003;18:1809–1819. [DOI] [PubMed] [Google Scholar]
- Rosen T, Kuczynski E, O'Neill LM, Funai EF, Lockwood CJ. Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J Matern Fetal Med 2001;10:297–300. [DOI] [PubMed] [Google Scholar]
- Rosen T, Schatz F, Kuczynski E, Lam H, Koo AB, Lockwood CJ. Thrombin-enhanced matrix metalloproteinase-1 expression: a mechanism linking placental abruption with premature rupture of the membranes. J Matern Fetal Neonatal Med 2002;11:11–17. [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, Schwartz TW. The chemokine system—a major regulator of angiogenesis in health and disease. APMIS 2004;112:481–495. [DOI] [PubMed] [Google Scholar]
- Rubens CE, Sadovsky Y, Muglia L, Gravett MG, Lackritz E, Gravett C. Prevention of preterm birth: harnessing science to address the global epidemic. Sci Transl Med 2014;6:262sr265. [DOI] [PubMed] [Google Scholar]
- Rudolph-Owen LA, Slayden OD, Matrisian LM, Brenner RM. Matrix metalloproteinase expression in Macaca mulatta endometrium: evidence for zone-specific regulatory tissue gradients. Biol Reprod 1998;59:1349–1359. [DOI] [PubMed] [Google Scholar]
- Runic R, Schatz F, Wan L, Demopoulos R, Krikun G, Lockwood CJ. Effects of norplant on endometrial tissue factor expression and blood vessel structure. J Clin Endocrinol Metab 2000;85:3853–3859. [DOI] [PubMed] [Google Scholar]
- Salafia CM, Lopez-Zeno JA, Sherer DM, Whittington SS, Minior VK, Vintzileos AM. Histologic evidence of old intrauterine bleeding is more frequent in prematurity. Am J Obstet Gynecol 1995;173:1065–1070. [DOI] [PubMed] [Google Scholar]
- Salamonsen LA, Butt AR, Hammond FR, Garcia S, Zhang J. Production of endometrial matrix metalloproteinases, but not their tissue inhibitors, is modulated by progesterone withdrawal in an in vitro model for menstruation. J Clin Endocrinol Metab 1997;82:1409–1415. [DOI] [PubMed] [Google Scholar]
- Salamonsen LA, Marsh MM, Findlay JK. Endometrial endothelin: regulator of uterine bleeding and endometrial repair. Clin Exp Pharmacol Physiol 1999;26:154–157. [DOI] [PubMed] [Google Scholar]
- Salker M, Teklenburg G, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C et al. . Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One 2010;5:e10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon C, Yee SW, Mitchell MD, Rice GE. The possible role of extravillous trophoblast-derived exosomes on the uterine spiral arterial remodeling under both normal and pathological conditions. Biomed Res Int 2014;2014:693157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez ER. Chaperoning steroidal physiology: lessons from mouse genetic models of Hsp90 and its cochaperones. Biochim Biophys Acta 2012;1823:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaper F, Rose-John S. Interleukin-6: biology, signaling and strategies of blockade. Cytokine Growth Factor Rev 2015;26:475–487. [DOI] [PubMed] [Google Scholar]
- Schatz F, Lockwood CJ. Progestin regulation of plasminogen activator inhibitor type 1 in primary cultures of endometrial stromal and decidual cells. J Clin Endocrinol Metab 1993;77:621–625. [DOI] [PubMed] [Google Scholar]
- Schatz F, Papp C, Toth-Pal E, Lockwood CJ. Ovarian steroid-modulated stromelysin-1 expression in human endometrial stromal and decidual cells. J Clin Endocrinol Metab 1994;78:1467–1472. [DOI] [PubMed] [Google Scholar]
- Schatz F, Papp C, Aigner S, Krikun G, Hausknecht V, Lockwood CJ. Biological mechanisms underlying the clinical effects of RU 486: modulation of cultured endometrial stromal cell stromelysin-1 and prolactin expression. J Clin Endocrinol Metab 1997;82:188–193. [DOI] [PubMed] [Google Scholar]
- Schatz F, Krikun G, Runic R, Wang EY, Hausknecht V, Lockwood CJ. Implications of decidualization-associated protease expression in implantation and menstruation. Semin Reprod Endocrinol 1999;17:3–12. [DOI] [PubMed] [Google Scholar]
- Schatz F, Krikun G, Caze R, Rahman M, Lockwood CJ. Progestin-regulated expression of tissue factor in decidual cells: implications in endometrial hemostasis, menstruation and angiogenesis. Steroids 2003;68:849–860. [DOI] [PubMed] [Google Scholar]
- Schoen CN, Tabbah S, Iams JD, Caughey AB, Berghella V. Why the United States preterm birth rate is declining. Am J Obstet Gynecol 2015;213:175–180. [DOI] [PubMed] [Google Scholar]
- Schonenberger MJ, Kovacs WJ. Hypoxia signaling pathways: modulators of oxygen-related organelles. Front Cell Dev Biol 2015;3:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secura GM, Madden T, McNicholas C, Mullersman J, Buckel CM, Zhao Q, Peipert JF. Provision of no-cost, long-acting contraception and teenage pregnancy. N Engl J Med 2014;371:1316–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey AM, Xiong S, Kennedy PR, Gardner L, Farrell LE, Chazara O, Ivarsson MA, Hiby SE, Colucci F, Moffett A. Tissue-specific education of decidual NK cells. J Immunol 2015;195:3026–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, Yoon BH. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 2004;191:1339–1345. [DOI] [PubMed] [Google Scholar]
- Short M, Dallay D, Omokanye S, Stauch K, Inki P. Acceptability of long-acting, progestin-only contraception in Europe: a two-year prospective, non-interventional study. Eur J Contracept Reprod Health Care 2014;19:29–38. [DOI] [PubMed] [Google Scholar]
- Singer CF, Marbaix E, Kokorine I, Lemoine P, Donnez J, Eeckhout Y, Courtoy PJ. The matrix metalloproteinase-1 (MMP-1) expression in the human endometrium is inversely regulated by interleukin-1 alpha and sex steroids. Ceska Gynekol 2000;65:211–215. [PubMed] [Google Scholar]
- Slater M, Quagliotto G, Cooper M, Murphy CR. Endometriotic cells exhibit metaplastic change and oxidative DNA damage as well as decreased function, compared to normal endometrium. J Mol Histol 2005;36:257–263. [DOI] [PubMed] [Google Scholar]
- Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol 2009;174:1959–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel BE, Schneider DJ. Platelet function, coagulopathy, and impaired fibrinolysis in diabetes. Cardiol Clin 2004;22:511–526. [DOI] [PubMed] [Google Scholar]
- Starzinski-Powitz A, Zeitvogel A, Schreiner A, Baumann R. In search of pathogenic mechanisms in endometriosis: the challenge for molecular cell biology. Curr Mol Med 2001;1:655–664. [DOI] [PubMed] [Google Scholar]
- Steel JH, O'Donoghue K, Kennea NL, Sullivan MH, Edwards AD. Maternal origin of inflammatory leukocytes in preterm fetal membranes, shown by fluorescence in situ hybridisation. Placenta 2005;26:672–677. [DOI] [PubMed] [Google Scholar]
- Storer CL, Dickey CA, Galigniana MD, Rein T, Cox MB. FKBP51 and FKBP52 in signaling and disease. Trends Endocrinol Metab 2011;22:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MX, Hu XH, Liu ZZ, Kwak-Kim J, Liao AH. What are the roles of macrophages and monocytes in human pregnancy? J Reprod Immunol 2015;112:73–80. [DOI] [PubMed] [Google Scholar]
- Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann N Y Acad Sci 2002;955:89–100; discussion 118, 396–406. [DOI] [PubMed] [Google Scholar]
- Taylor RN, Kane MA, Sidell N. Pathogenesis of endometriosis: roles of retinoids and inflammatory pathways. Semin Reprod Med 2015;33:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecchio C, Cassatella MA. Neutrophil-derived cytokines involved in physiological and pathological angiogenesis. Chem Immunol Allergy 2014;99:123–137. [DOI] [PubMed] [Google Scholar]
- Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol 2014;5:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatipamula S, Hossain MA. Critical role of extracellularly secreted neuronal pentraxin 1 in ischemic neuronal death. BMC Neurosci 2014;15:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Augustin HG. The role of the angiopoietins in vascular morphogenesis. Angiogenesis 2009;12:125–137. [DOI] [PubMed] [Google Scholar]
- Thurston G, Daly C. The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold Spring Harb Perspect Med 2012;2:a006550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepicchio WL, Bozza M, Pedneault G, Dorner AJ. Recombinant human IL-11 attenuates the inflammatory response through down-regulation of proinflammatory cytokine release and nitric oxide production. J Immunol 1996;157:3627–3634. [PubMed] [Google Scholar]
- Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab 2003;88:5555–5563. [DOI] [PubMed] [Google Scholar]
- Uz YH, Murk W, Bozkurt I, Kizilay G, Arici A, Kayisli UA. Increased c-Jun N-terminal kinase activation in human endometriotic endothelial cells. Histochem Cell Biol 2011;135:83–91. [DOI] [PubMed] [Google Scholar]
- Varney SJ, Guest JF. Relative cost effectiveness of Depo-Provera, Implanon, and Mirena in reversible long-term hormonal contraception in the UK. Pharmacoeconomics 2004;22:1141–1151. [DOI] [PubMed] [Google Scholar]
- Veerbeek JH, Post Uiterweer ED, Nikkels PG, Koenen SV, van der Zalm M, Koster MP, Burton GJ, van Rijn BB, Franx A. Biopsy techniques to study the human placental bed. Placenta 2015;36:775–782. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lin M, Weng H, Wang X, Yang L, Liu F. ENMD-1068, a protease-activated receptor 2 antagonist, inhibits the development of endometriosis in a mouse model. Am J Obstet Gynecol 2014;210:531 e531–538. [DOI] [PubMed] [Google Scholar]
- Weich NS, Wang A, Fitzgerald M, Neben TY, Donaldson D, Giannotti J, Yetz-Aldape J, Leven RM, Turner KJ. Recombinant human interleukin-11 directly promotes megakaryocytopoiesis in vitro. Blood 1997;90:3893–3902. [PubMed] [Google Scholar]
- Weisberg E, Bateson D, McGeechan K, Mohapatra L. A three-year comparative study of continuation rates, bleeding patterns and satisfaction in Australian women using a subdermal contraceptive implant or progestogen releasing-intrauterine system. Eur J Contracept Reprod Health Care 2014;19:5–14. [DOI] [PubMed] [Google Scholar]
- Weiss JL, Malone FD, Vidaver J, Ball RH, Nyberg DA, Comstock CH, Hankins GD, Berkowitz RL, Gross SJ, Dugoff L et al. . Threatened abortion: a risk factor for poor pregnancy outcome, a population-based screening study. Am J Obstet Gynecol 2004;190:745–750. [DOI] [PubMed] [Google Scholar]
- Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J 1999;13:781–792. [PubMed] [Google Scholar]
- Wewer UM, Faber M, Liotta LA, Albrechtsen R. Immunochemical and ultrastructural assessment of the nature of the pericellular basement membrane of human decidual cells. Lab Invest 1985;53:624–633. [PubMed] [Google Scholar]
- Wu ZM, Yang H, Li M, Yeh CC, Schatz F, Lockwood CJ, Di W, Huang SJ. Pro-inflammatory cytokine-stimulated first trimester decidual cells enhance macrophage-induced apoptosis of extravillous trophoblasts. Placenta 2012;33:188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–1136. [DOI] [PubMed] [Google Scholar]
- Zaga-Clavellina V, Garcia-Lopez G, Flores-Pliego A, Merchant-Larios H, Vadillo-Ortega F. In vitro secretion and activity profiles of matrix metalloproteinases, MMP-9 and MMP-2, in human term extra-placental membranes after exposure to Escherichia coli. Reprod Biol Endocrinol 2011;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakar T, Hertelendy F. Progesterone withdrawal: key to parturition. Am J Obstet Gynecol 2007;196:289–296. [DOI] [PubMed] [Google Scholar]
- Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol 2002;160:1405–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Luo X, Qi HB, Zong WJ, Zhang H, Liu DD, Li QS. The expression of pentraxin 3 and tumor necrosis factor-alpha is increased in preeclamptic placental tissue and maternal serum. Inflamm Res 2012;61:1005–1012. [DOI] [PubMed] [Google Scholar]
- Zimna A, Kurpisz M. Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: applications and therapies. Biomed Res Int 2015;2015:549412. [DOI] [PMC free article] [PubMed] [Google Scholar]