Figure 2.
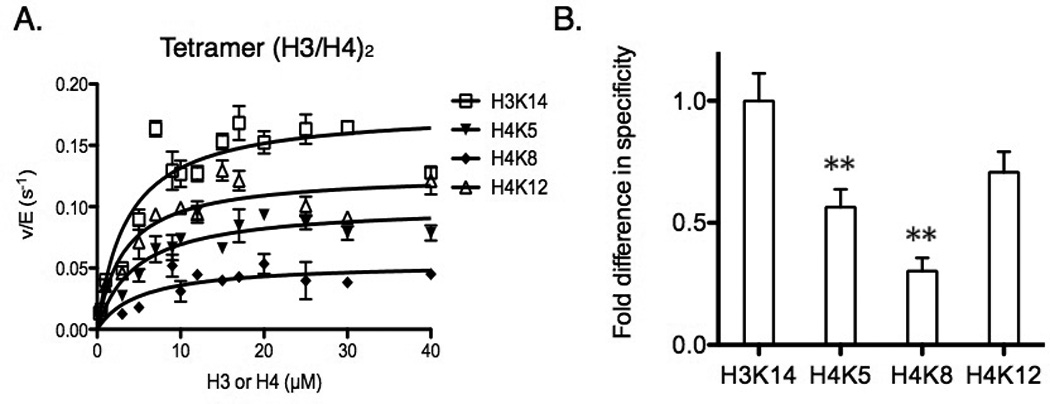
Specificity comparison between lysine residues in (H3/H4)2. (A) Determination of steady-state parameters for individual lysines (H3K14, H4K5, H4K8, and H4K12) of (H3/H4)2 acetylation catalyzed by Piccolo NuA4 in the presence of saturating acetyl-CoA. The error bar represents the standard error of v/E. The apparent kinetic parameters are summarized in Table 2. (B) The fold difference in specificity is determined by the ratio of kcat(app), and each site is normalized to the highest kcat(app) of H3K14. ** p < 0.01 when compared to H3K14. The specificity for H3K14 and H4K12 are not significantly different. The error bar represents the standard error of specificity measurements.
