Figure 4.
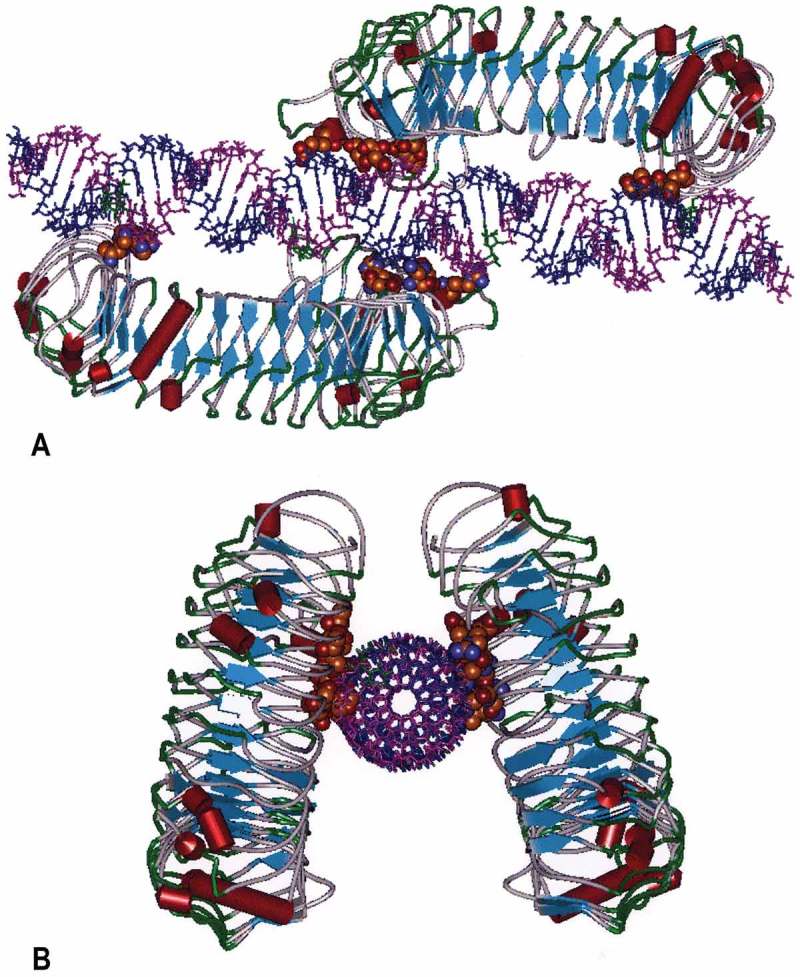
Molecular Model of the human TLR3 dimer ecodomain and its rintatolimod ligand. Figure 4(a) is viewed from a lateral view of rintatolimod bound to the active site of the TLR3 homodimer. The C-terminal regions of each dimer face each other and bind to the phosphate backbone of the dsRNA. The N-terminals of each TLR3 bind to opposite ends of the dsRNA with a minimum length of 45 bp required for interaction with essential residues of TLR3 for activation of intracellular signaling. Amino acids of TLR3 required for binding of rintatolimod are shown as CPK (Van der Waals’ radii) associated with the phosphate backbone. Figure 4(b) illustrates the TLR3 homodimer complexed with rintatolimod as seen down the long axis of the dsRNA. The TLR3 homodimers are represented as structural elements with the blue arrows signifying direction of β-sheets and the red cylinders signifying α-helices. The Poly I strand of rintatolimod is colored blue and the poly C12U strand magenta. Reproduced from Mitchell WM, et al. Discordant Biological and Toxicological Species Responses to TLR3 Activation. Am J Path 2014; 184: 1062–72.
