Fig. 2.
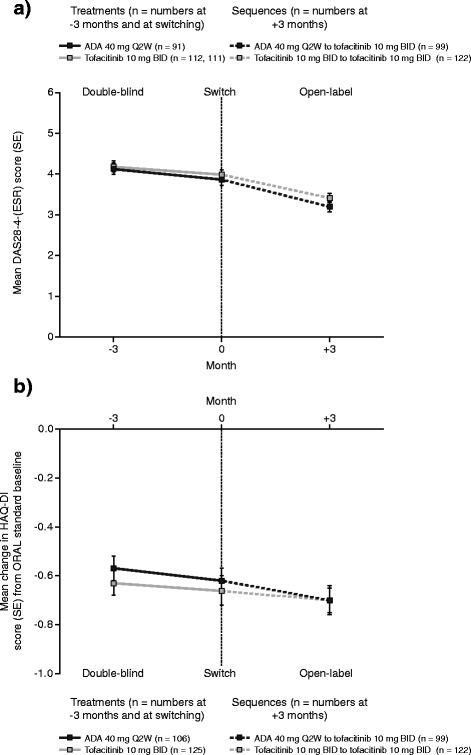
Disease Activity Score for 28-joint counts based on the erythrocyte sedimentation rate (DAS28-4(ESR)) and changes from baseline in the Health Assessment Questionnaire-Disability Index (HAQ-DI) 3 months before switch, at switch, and 3 months after switch*. ADA adalimumab, BID twice daily. *Includes patients who completed treatment with ADA 40 mg Q2W or tofacitinib 10 mg BID in ORAL Standard (or discontinued treatment for reasons other than a tofacitinib-related serious AE) and then enrolled in ORAL Sequel and switched treatment with minimal washout (≤2 weeks after their last dose of study drug in ORAL Standard). Lower scores indicate less disease activity (DAS28–4[ESR]) or lower disability (HAQ-DI). ADA, adalimumab; BID, twice daily; DAS28–4(ESR), Disease Activity Score for 28 joint counts based on the erythrocyte sedimentation rate; HAQ-DI, Health Assessment Questionnaire-Disability Index; Q2W, every 2 weeks; SE, standard error. (a) Changes from Baseline in Disease Activity Score for 28-joint counts based on Erythrocyte sedimentation rate (DAS28-4(ESR) 3 months before switch, at switch, and 3 months after switch.* (b) Changes from Baseline in the Health Assessment QUestionnaire-Disability Index (HAQ-DI) 3 months before switch, at switch, and 3 months after switch*
