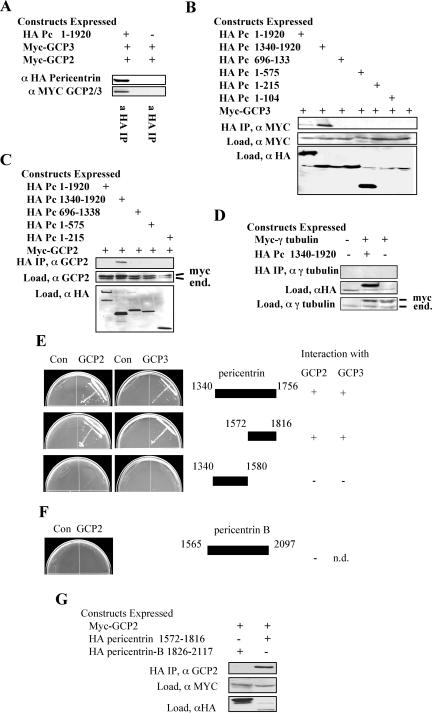
Figure 3.
C-terminal domains of pericentrin interact with γ TuRC proteins GCP3 and GPC2 in vitro. (A) When coexpressed in vertebrate cells myc-tagged GCP2 and/or myctagged GCP3 coimmunoprecipitate with HA-tagged pericentrin (similar mobility of GCP2 and GCP3 prevents their individual identification in this experiment). (B–D) A C-terminal domain of pericentrin (amino acids 1340–1920) interacts with GCP3 (B) and GCP2 (C) but not γ tubulin (D) when coexpressed in vertebrate cells. Immunoprecipitation and immunoblotting performed as indicated. End., endogenous protein. (E) Two-hybrid analysis confirms the interaction of the pericentrin C terminus with GCP2 and GCP3 but not with γ tubulin (data not shown). (F and G) Segments of pericentrin-B corresponding to the C terminal region of pericentrin do not interact with GCP2 in two-hybrid (F) or coimmunoprecipitation experiments (G).