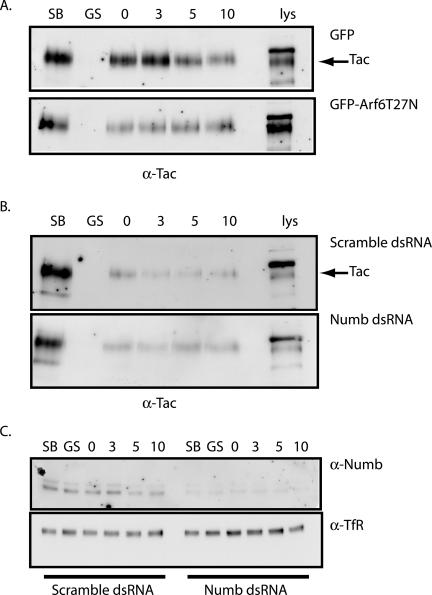
Figure 9.
Numb functions in regulation of Tac recycling. (A) CHO cells cotransfected with pTac and either GFP-Arf6-T27N or pEGFP control vector were surface-biotinylated on ice for 40 min. One plate was removed to depict total surface biotinylation (lane SB), and another was stripped with glutathione to ensure efficient removal of biotin label (lane GS). The cells were then allowed to internalize for 60 min at 18°C and returned to ice to strip any label remaining at the surface. After glutathione stripping, cells were returned to 37°C for chase periods 0, 3, 5, and 10 min. At each chase time point, cells were glutathione-stripped, and remaining biotinylated proteins were recovered from equal amounts of protein lysates using streptavidin beads and were analyzed by SDS-PAGE. Tac receptor was detected by immunoblotting. Arrow indicates band corresponding to the Tac receptor. (B) Similar to experiment above, CHO cells were cotransfected with pTac and either double-stranded RNA duplex specific for Numb (bottom panel) or with a scrambled RNA duplex (top panel), and intracellular Tac receptor levels were monitored by surface biotinylation. Arrow indicates band corresponding to the Tac receptor. (C) Equal amounts of protein lysates were analyzed by SDS-PAGE to confirm depletion of endogenous Numb levels by immunoblotting for Numb (top panel). The blots were then stripped and reprobed for endogenous transferrin receptor to confirm equal loading in of each condition (α-TfR, bottom panel).