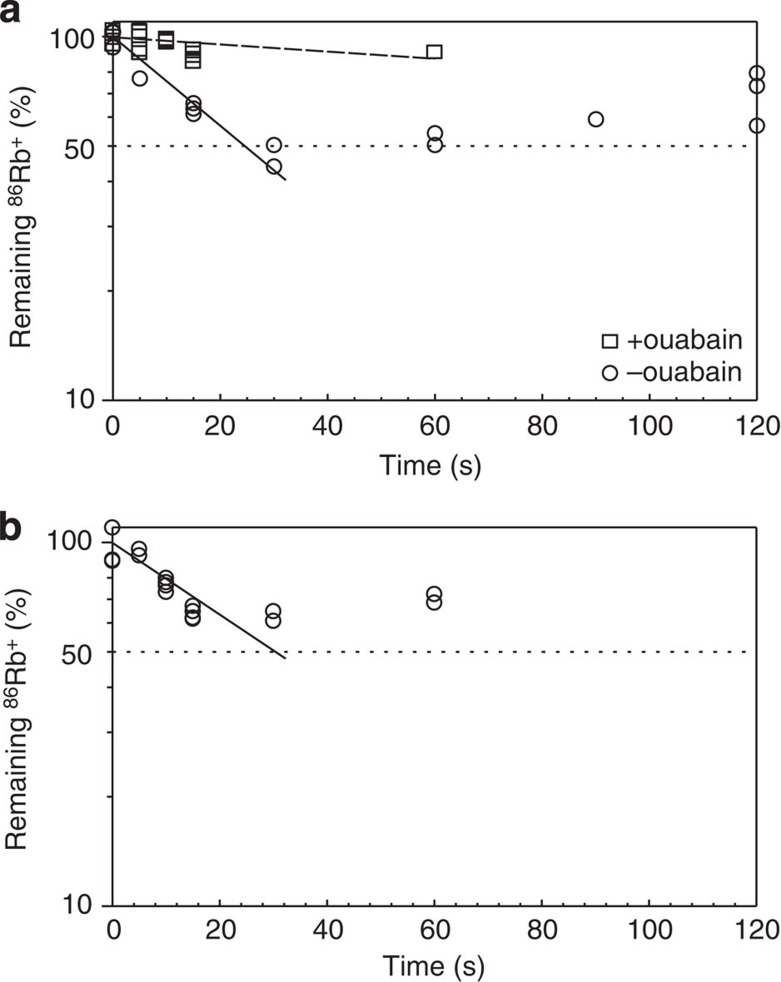
Figure 2. Isotopic measurements of the substitution of bound 86Rb+ with K+ congeners.
Substitution of 86Rb+ in shark rectal gland Na+,K+-ATPase in E2·MgF42−·2Rb+ with Rb+ (a) or Tl+ (b) at 20 oC. The lines represent single exponential fits to the measurements in the absence of ouabain (circles) up to 30 s with time constants of 14±1 s (a) and 17±2 s (b), assuming that only half of the bound Rb+ is substituted. Broken line and squares in a show Rb+ substitution with the preformed ATPase–ouabain complex measured after 2 h of incubation. In the normalization of the data, zero is taken at 10%, corresponding to the dilution of 86Rb+ in the dissociation buffer. Note that substitution ceases within 30 s when approximately half of the initially bound 86Rb+ has been substituted.