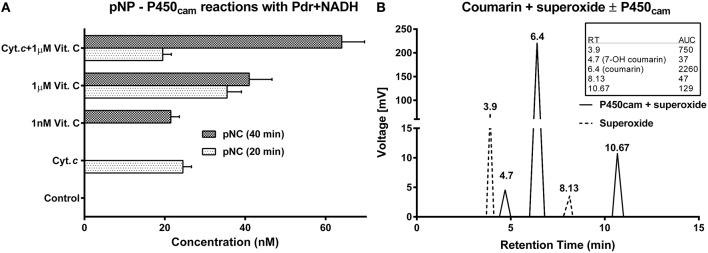
Figure 6.
Demonstration of nonspecific partnering with diverse combinations of P450cam, reductases, auxiliary redox partners, substrates and electron donors: All the reactions were carried out at 27 ± 1°C in 100 mM potassium phosphate buffer (pH 7.4). The initial concentration of substrate employed was 200 μM. Enzyme concentrations were as follows: [P450cam] = 0.5 μM and [Pdr] = 0.6 μM. Electron donors: [NADH] and [H2O2] = 1 mM, [O] = 50 μM. [Cyt. c] and [Vit. C] = 1 μM. (A) P450cam reactions with pNP (a CYP2E1 marker substrate), employing Pdr and NADH as redox equivalent generating agents. The control had only pNP, P450cam, Pdr, and NADH. (B) Schematic of HPLC profiles obtained with P450cam and coumarin (a CYP2B6 substrate), employing superoxide as the sole redox agent. The inset to the right shows the original area under curve (AUC) values obtained.