Figure 4.
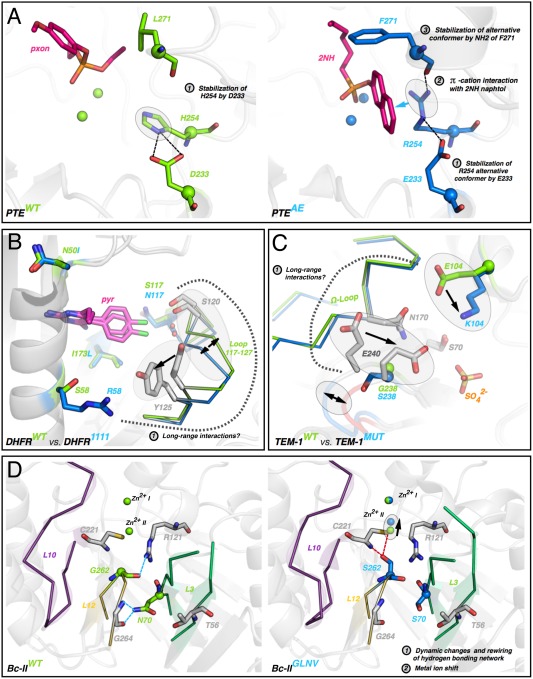
Molecular mechanisms underlying strong cases of epistasis. (A) Direct interactions between mutations in PTE. The first round mutation H254R directly interacts with the 2NH substrate (magenta sticks). Then subsequent mutations D233E/L271F directly stabilize the position of R254 in PTEAE (evolved mutant, right). (B) Indirect interaction between R58S and S117N in DHFR. R58 may cause a shift of loop 117‐127, which may reposition N117 and S120, emphasizing the steric clash with pyrimethamine (pyr, magenta sticks). (C) Indirect interactions between E104K and G238S in TEM‐1. The two mutations may interact through the Ω‐loop and the shift of E240 that is likely contacting the substrate. (D) Indirect interactions between mutations G262S and N70S originate from a rewiring of the hydrogen‐bonding network between loops L12 and L3, causing a displacement of a catalytic zinc ion.
