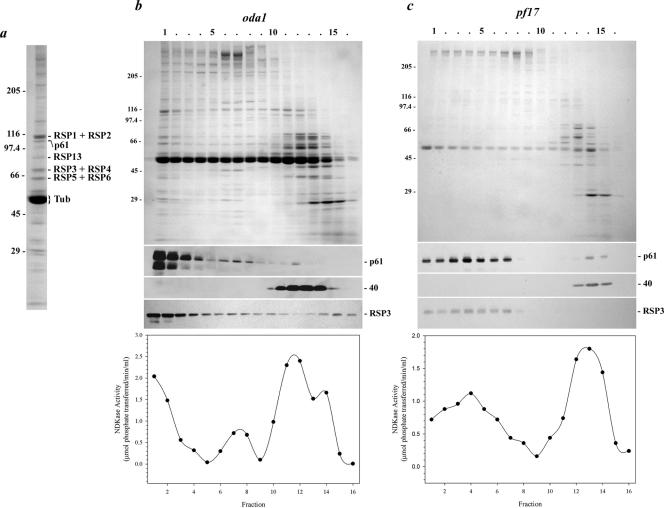
Figure 7.
p61 copurifies with radial spoke protein 3.(a) Coomassie Blue-stained lane of isolated radial spokes (from the sucrose gradient shown in b). The p61 protein and several RSP components were identified by immunoblot analysis and/or by comparison with the electrophoretic analysis performed by Yang et al. (2001). (b) Axonemes from the mutant oda1 were treated with 0.6 M NaCl to remove inner arm dyneins, and the radial spokes were subsequently extracted with 0.5 M KI. This extract was then sedimented in a 5–20% sucrose density gradient. Fractions were electrophoresed in a 5–15% acrylamide gradient gel and either stained with Coomassie Blue (top) or blotted to nitrocellulose and probed with CT220 (middle) and the RSP3 antibody (bottom). Most of the p61 and RSP3 proteins were found together in fractions 1–3. In contrast, the Mr 40,000 band detected by antibody CT220 sedimented near the top of the gradient in fractions 11–14. NDKase activity of each fraction is shown in the lower panel, and precisely copurified with p61 near the bottom of the gradient and also with p40 toward the top of the gradient. (c) Radial spokes lacking the spoke head were extracted from pf17 axonemes and sedimented in a 5–20% sucrose gradient. Both p61 and RSP3 were present nearer to the top of the gradient in fractions 1–7; sedimentation of the Mr 40,000 band was unaffected by the pf17 mutation. NDKase activity also was shifted up the gradient and again followed the p61 profile.