Abstract
Aerobic anoxygenic phototrophic (AAP) bacteria are photoheterotrophs that despite their low abundances have been hypothesized to play an ecologically and biogeochemically important role in aquatic systems. Characterizing this role requires a better understanding of the in situ dynamics and activity of AAP bacteria. Here we provide the first assessment of the single-cell activity of freshwater AAP bacteria and their contribution to total bacterial production across lakes spanning a wide trophic gradient, and explore the role of light in regulating AAP activity. The proportion of cells that were active in leucine incorporation and the level of activity per cell were consistently higher for AAP than for bulk bacteria across lakes. As a result, AAP bacteria contributed disproportionately more to total bacterial production than to total bacterial abundance. Interestingly, although environmentally driven patterns in activity did not seem to differ largely between AAP and bulk bacteria, their response to light did, and exposure to light resulted in increases in the proportion of active AAP bacteria with no clear effect on their cell-specific activity. This suggests that light may play a role in the activation of AAP bacteria, enabling these photoheterotrophs to contribute more to the carbon cycle than suggested by their abundance.
Introduction
Aerobic anoxygenic phototrophic (AAP) bacteria are a cosmopolitan photoheterotrophic group of prokaryotes that inhabit the water column of all aquatic ecosystems (Yurkov and Csotonyi, 2009; Koblížek, 2015). The capacity of AAP bacteria to obtain energy from both the oxidation of organic matter and light-induced proton translocation has led to explorations of the potential competitive advantage of AAP bacteria and their trophic and biogeochemical roles in aquatic ecosystems (Moran and Miller, 2007; Béjà and Suzuki, 2008; Ferrera et al., 2011). Experimental studies with AAP bacterial isolates demonstrate that light stimulates biomass production and increases the efficiency of utilization of organic carbon sources (Biebl and Wagner-Döbler, 2006; Spring et al., 2009; Hauruseu and Koblížek, 2012), suggesting that AAP cells benefit from the capacity to use light-derived energy. However, most in situ studies on the ecology of AAP bacteria have focused on exploring their abundance or diversity (Koblížek, 2015), and very little is known about the physiological status of AAP cells in natural aquatic environments, and whether and how sunlight influences the in situ dynamics and activity of AAP bacteria. This largely limits our understanding of the ecological importance of this group, and the potential global significance of the photoheterotrophic processes carried out by AAP bacteria in natural aquatic ecosystems.
Available data indicate that despite their low relative abundances and the apparently low amount of energy potentially gained through phototrophy (Kirchman and Hanson, 2013), AAP bacteria are more active, larger and grow faster than the bulk bacterial community (Koblízek et al., 2007; Ferrera et al., 2011; Hojerová et al., 2011; Kirchman et al., 2014). It is not clear, however, whether these higher apparent growth rates and potential competitiveness of AAP bacteria can be directly attributed to a light-driven effect. Recently, Stegman et al. (2014) developed an approach that combines infrared epifluorescence microscopy with microautoradiography (AAP-MAR) in order to directly assess the activity of AAP bacteria from natural communities by measuring the incorporation of radioactive substrates by individual AAP cells. Recent studies using this approach in the Delaware estuary and the coastal waters of the West Antarctic Peninsula (Kirchman et al., 2014; Stegman et al., 2014) confirmed that AAP cells were on average more active in substrate uptake than bulk bacterial cells, although there were large spatial and seasonal variations in the percentage of active AAP bacteria. Moreover, contrary to expectations, light did not enhance AAP single-cell activity in leucine incorporation in experimental incubations relative to dark controls, although AAP activity was positively correlated with light availability in the water column. The evidence converges to suggest that in terms of activity, AAP bacteria must play a role that is disproportionately large relative to their biomass in aquatic systems, yet we are still far from understanding under what circumstances this role may be more or less important, under what scenarios this group has a competitive advantage relative to other prokaryotes and how light influences the activity of this group of prokaryotes. In addition, all studies of AAP bacterial activity in natural environments have been carried out in estuarine or marine waters, and the magnitude and regulation of AAP bacterial activity in freshwater ecosystems remain completely unknown.
In this paper, we explore patterns in single-cell activity of freshwater AAP bacteria across a range of temperate lakes in Québec (Canada) that vary widely in trophic status and light availability. Using the AAP-MAR approach developed by Stegman et al. (2014), we investigated how the proportion of active AAP bacteria and their single-cell activity in leucine incorporation vary among lakes, and how they compare with activity patterns of the bulk bacterial community. In addition, we explored the role of light in regulating the heterotrophic activity of bulk and AAP bacteria by exposing lake samples to artificial photosynthetically active radiation (PAR). By quantifying the area of silver grains associated with active cells and comparing it with bulk bacterial production rates, we were able to estimate, for the first time, the contribution of freshwater AAP bacteria to total bacterial biomass production in their natural setting.
Materials and methods
Study sites and sample collection
We sampled 7 northern temperate lakes, 6 of which were located in the Eastern Townships region of southeastern Québec (45.24°N, 72.12°W) and one in the Laurentian region north of Montréal (46.01°N, 74.15°W), Canada (Table 1). The lakes were chosen to cover a wide range in dissolved organic carbon (DOC) concentrations and a gradient in lake productivity, both in terms of chlorophyll a (Chl a) and nutrient concentrations, so that they differed greatly in trophic status and light availability (Table 1). Each lake was sampled once between July and August 2013. During summer stratification, lakes may develop a hypoxic hypolimnion that makes the detection of AAP bacteria problematic, as it is not possible to distinguish bacteriochlorophyll a (BChl a)-containing AAP bacteria from BChl a-containing anaerobic phototrophic cells. In order to avoid sampling in hypoxic zones, we measured vertical profiles of temperature and dissolved oxygen for all lakes and collected water samples from the aerobic epilimnetic layer. These profiles were recorded with a Yellow Springs Instruments (YSI, Yellow Springs, OH, USA) Pro Plus multiparameter probe and underwater profiles of PAR were measured with a LI-COR LI-190 Quantum Sensor (Lincoln, NE, USA).
Table 1. Environmental properties, prokaryotic abundances, activity and BChl a concentrations and content in the epilimnetic waters of the lakes studied.
| Environmental properties | Bowker | Waterloo | Magog | Coulombe | Aylmer | Nicolet | Croche |
|---|---|---|---|---|---|---|---|
| Maximum depth (m) | 63 | 6 | 11 | 9 | 14 | 29 | 11 |
| Sampling depth (m) | 6.0 | 2.0 | 4.0 | 0.5 | 1.0 | 3.5 | 1.5 |
| KPAR (m−1) | 0.31 | 1.34 | 0.50 | 1.52 | 1.05 | 0.52 | 0.67 |
| Temperature (°C) | 21 | 25 | 23 | 19 | 20 | 20 | 21 |
| Dissolved oxygen (%) | 110 | 103 | 108 | 89 | 95 | 94 | 96 |
| pH | 8.2 | 8.6 | 8.5 | 7.5 | 7.2 | 7.5 | 6.7 |
| DOC (mg l−1) | 2.7 | 7.8 | 4.9 | 14 | 10 | 3.5 | 4.8 |
| TP (μg l−1) | 3.3 | 7.3 | 12.7 | 16.0 | 11.7 | 2.7 | 2.7 |
| DP (μg l−1) | 2.5 | 6.2 | 5.5 | 8.4 | 7.3 | 1.6 | 1.3 |
| TN (mg l−1) | 0.15 | 0.49 | 0.25 | 0.44 | 0.45 | 0.29 | 0.16 |
| DN (mg l−1) | 0.13 | 0.48 | 0.21 | 0.37 | 0.41 | 0.28 | 0.15 |
| Chl a (μg l−1) | 0.8 | 11.0 | 2.9 | 3.7 | 3.3 | 0.7 | 1.1 |
| Bacterial characteristics | |||||||
| Total bacteria (106 cells per ml) | 2.65 | 5.27 | 3.43 | 2.42 | 2.98 | 1.38 | 0.92 |
| AAP bacteria (104 cells per ml) | 3.05 | 13.5 | 7.78 | 6.71 | 9.57 | 4.16 | 4.00 |
| AAP bacteria (% of DAPI counts) | 1.15 | 2.57 | 2.27 | 2.78 | 3.22 | 3.01 | 4.34 |
| BChl a (ng l−1) | 5.94 | 24.29 | 10.52 | 9.80 | 5.16 | 8.53 | 12.57 |
| Specific BChl a (10−1 fg per cell) | 1.95 | 1.80 | 1.35 | 1.46 | 0.54 | 2.05 | 3.14 |
Abbreviations: AAP, aerobic anoxygenic phototrophic; BChl a, bacteriochlorophyll a; Chl a, chlorophyll a; DAPI, 4',6-diamidino-2-phenylindole; DN, dissolved nitrogen; DOC, dissolved organic carbon; DP, dissolved phosphorous; KPAR, attenuation coefficient of photosynthetically active radiation; TN, total nitrogen; TP, total phosphorous.
Lake samples were collected by pumping water into 20 l polycarbonate containers from a depth corresponding to an irradiance in the range of 200–300 μmol photons m–2 s–1 and transported refrigerated in the dark to the laboratory. Microautoradiographic incubations were carried out immediately upon returning to the laboratory (2 to 3 h after collection). The relative abundance of AAP bacteria was estimated on fixed samples by standard infrared epifluorescence microscopy (Cottrell et al., 2006). AAP and total bacterial cell size was also determined by image analysis from the 4',6-diamidino-2-phenylindole (DAPI)-stained cells using the integration method (Sieracki et al., 1989).
Concentrations of total and dissolved phosphorous (TP, TDP) were measured by the molybdenum-blue method following persulfate digestion. Total and dissolved nitrogen (TN, TDN) were measured as nitrates after alkaline persulfate digestion. DOC was measured in 0.45 μm filtered samples by wet oxidation using an OI Analytical Total Carbon Analyzer (College Station, TX, USA).
Chl a and BChl a concentrations
Water samples were filtered through GF/F filters (Whatman GE, Mississauga, ON, Canada), frozen and subsequently extracted with hot ethanol. Chl a in the extracts was measured spectrophotometrically at 665 nm using an UV/Vis UltroSpec 2100 Pro spectrophotometer (Biochrom, Holliston, MA, USA), correcting for turbidity at 750 nm and for the presence of pheophytin. BChl a concentrations were measured on a FL3500-FT fluorometer (Photon Systems Instruments, Kolackova, Czech Republic) using the standard calibration curve provided by the manufacturer. Samples were first treated with 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU, 50 μM final concentration), an inhibitor of photosystem II in oxygenic phototrophs, so that BChl a fluorescence could be distinguished from Chl a fluorescence (Koblízek et al., 2005).
Microautoradiography of AAP and total bacteria
The single-cell activity of AAP bacteria and bulk bacteria was determined using the AAP-MAR method, as described previously (Stegman et al., 2014). In brief, 30 ml water samples were spiked with 3H-leucine (20 nM final concentration) in 60 ml transparent polystyrene culture flasks, and incubated as described below. Incubations were ended with the addition of paraformaldehyde (2% final concentration), the samples were filtered onto 0.2 μm pore size black polycarbonate filters and then stored at −80 °C for subsequent analysis. Sections (1/8) of filters were mounted on slides and first examined by infrared epifluorescence microscopy to determine the exact location of microscope fields containing AAP cells. After identifying and counting the AAP cells, the filter sections were then taken off the microscope and subjected to the microautoradiography procedure as described by Cottrell and Kirchman (2003). A time series of autoradiographic exposures was used to select the shortest time required to identify the maximum number of active cells in each lake sample. The exposure time was 7 days for all samples except for the oligotrophic Lake Bowker, for which it was 35 days. After exposure, slides were developed and fixed, dried overnight and the filter sections were carefully peeled off of the emulsion so that the cells remained stuck to the slide. The cells were then stained with DAPI, and the previously analyzed fields were relocated automatically under the epifluorescence microscope and analyzed again for the presence of silver grains around DAPI-stained cells. After analyzing all fields of view, ImagePro (Media Cybernetics, Rockville, MD, USA) was used to align DAPI images with the identified AAP cells from before and after the autoradiographic exposure. If the images matched, the two DAPI images were merged to create a composite image and the MicrobeCounter program (Cottrell and Kirchman, 2003) was used to count AAP and total bacteria, to quantify those with silver grains and to estimate the size of the silver grain area (SGA) around active cells. As microscopic counts of total bacteria include AAP bacteria, any comparison between AAP and total bacteria would be conservative even though AAP make up only a small fraction of the total community (see Results).
Effect of light on single-cell activity and production of AAP and total heterotrophic bacteria
In order to evaluate the influence of light on the single-cell activity of AAP and total bacteria, AAP-MAR was applied to lake water samples that were incubated either under artificial PAR or in the dark. 3H-leucine incubations were conducted in 60 ml transparent polystyrene culture flasks. Three dark, three light flasks plus one paraformaldehyde-killed control were attached to a surface-tethered array and incubated under artificial light at an irradiance of 150 μmol photons m−2 s−1 for 4 h in a tank with circulating water to keep sample temperature at 20±2 °C. Illumination was provided by UV-free fluorescent lamps (45-W Sun Blaster, Kelowna, BC, Canada). The light intensity was measured with a HR 4000 spectrometer PAR sensor (Ocean Optics, Winter Park, FL, USA). The paraformaldehyde-fixed samples were filtered onto 0.2 μm pore size black polycarbonate filters and stored at −80 °C for subsequent AAP-MAR analysis (see above).
To evaluate the influence of light on bulk bacterial production, three replicates of 1.5 ml of lake water plus one paraformaldehyde-killed control were inoculated with 3H-leucine at a final concentration of 20 nM and incubated for 2 h either in the dark or under the same light and temperature conditions described above. Incubations were terminated by the addition of paraformaldehyde (2% final concentration) and the samples were stored at 4 °C until processing by the microcentrifuge method (Smith and Azam, 1992).
Water color differed greatly among lakes, and thus we corrected for the possible disparity between the incident radiation and the actual incident light reaching the cells during the incubations. Measurements of incident radiation (I0) and the extinction coefficient (kd) of the lake water were used to calculate light intensity at the 1 cm depth of the water sample inside the incubation flask (ID), and the cell activity data obtained from the light incubations (percentage active cells, SGA and rate of leucine incorporation) were corrected with the coefficient that relates I0 and ID.
Statistical analyses
Differences in activity between AAP bacteria and bulk bacteria and differences between light and dark experiments were assessed with the Kruskal–Wallis nonparametric test or the paired t-test. Relative AAP bacterial abundance and percentages of active cells were arcsine transformed and SGAs were log transformed for parametric statistical analyses. Relationships between variables were investigated with linear correlation and regression techniques. All statistical analyses were performed using the JMP 9.0 statistical package (SAS Institute Inc., Cary, NC, USA).
Results
We explored variations in abundance, cell size and single-cell activity of AAP bacteria using the AAP-MAR approach in seven different temperate lakes (Table 1). The attenuation coefficient of PAR (KPAR) varied fourfold (0.3 to 1.5 m−1), DOC concentration varied fivefold (2.6 to 14 mg l−1) and Chl a and TP concentrations varied 15-fold (0.7 to 11 μg l−1) and sixfold (2.7 to 16 μg l−1), respectively, among the lakes (Table 1). All lakes except the shallow Lake Waterloo were stratified during the sampling period. Epilimnetic water temperatures averaged 21±2 °C. Lakes Bowker, Nicolet, Waterloo and Aylmer had a fully aerobic water column, whereas lakes Magog, Coulombe and Croche had an anoxic hypolimnion at the time of sampling. The high thermal stability in the latter three lakes during the time of sampling almost certainly prevented mixing events between the oxygenated surface layers and the deeper, anoxic layers. Considering that samples were always collected from fully aerobic subsurface waters, we can assume that the BChl a-containing bacteria and the BChl a concentrations observed originate from AAP bacteria, rather than from anaerobic phototrophic bacteria.
AAP and total bacterial abundance, cell size and single-cell activity
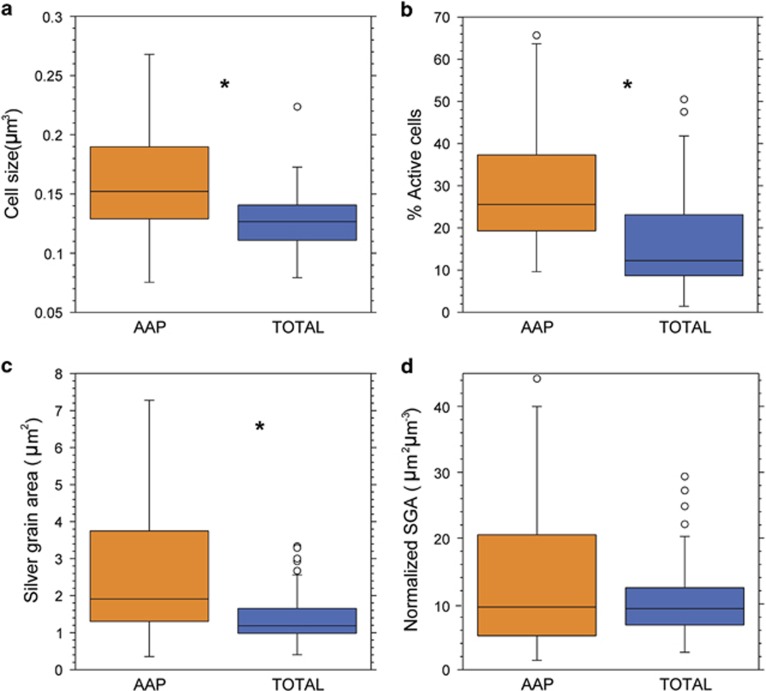
In general, AAP bacterial abundances were low and varied fourfold (3 × 104–1 × 105 cell per ml) among the lakes (Table 1). AAP abundance covaried with total prokaryotic abundance, thus resulting in small variations in the proportion of AAP bacteria among lakes (1% to 4% of total DAPI counts, mean 2.4%, Table 1). Total bacterial cell size varied 1.5-fold among all samples, from 0.10 to 0.18 μm3 (mean 0.13±0.036 μm3), whereas AAP bacterial cell size varied more than twofold (Figure 1a), from 0.13 to 0.29 μm3 (mean 0.17±0.056 μm3). The mean cell size of an AAP cell was 1.5-fold larger than that of an average cell in the total bacterial community (paired t-test, P<0.0001).
Figure 1.
Cell size (a), percentage of active cells (b), average SGA around active cells (c) and average SGA around active cells normalized for biovolume (d) of AAP and bulk bacterial cells. Central lines indicate median values and combine data from light and dark incubations, boxes indicate the lower and upper quartiles, whiskers depict the 10th and 90th percentiles and the dots represent outliers. The asterisks indicate significant differences (P<0.05) between AAP and total cells.
When all the lakes were considered together, the proportion of active cells was significantly higher for AAP bacteria than for total bacteria (paired t-test, combining light and dark incubations P<0.0001, Figure 1b). The percentage of cells actively taking up 3H-leucine varied remarkably among lakes, ranging from 2% to 44% of total bacteria and from 16% to 58% of AAP bacteria. Overall, the active fraction of AAP bacteria was almost twofold higher than the active fraction of the whole community.
As the level of activity per cell can also vary greatly, we also quantified the size of the SGA surrounding active cells. The SGA varied considerably among lake samples, with coefficients of variation of 49% and 72% for total bacteria and AAP cells, respectively. The mean SGA around active AAP cells was on average significantly larger than that for the bulk bacteria (paired t-test combining light and dark incubations, P<0.0001, Figure 1c). Overall, the mean SGA around AAP cells (2.6 μm2) was on average almost twofold larger than the area around active cells from the bulk bacterial community (1.44 μm2). There was no relationship between the mean cell size and the level of cell activity for AAP or total bacteria (data not shown), although it should be noted that the current version of the AAP-MAR method does not allow to discriminate between the cell sizes of active and inactive bacteria (Stegman et al., 2014). In order to account for the differences in size between AAP and total bacterial cells, the level of activity per cell (mean SGA) was normalized to the mean cell size (Figure 1d). The mean SGA per unit cell volume of an active AAP bacterium was slightly higher than that of an active cell in the total bacterial community (11 vs 16 μm2 μm−3 for total bacteria and AAP, respectively), although this difference was not statistically significant (P>0.05; paired t-test combining light and dark incubations).
The variability in bacterial activity among replicate samples differed from lake to lake. The mean s.e. of three replicate microautoradiographic assays ranged from 0.20% to 6.84% for total bacteria and from 1.45% to 18.1% for AAP bacteria (Supplementary Table S1). The s.e. associated with the measurements of SGA in three replicate samples ranged from 0.02 to 0.53 μm2 for active cells in the bulk bacterial community and from 0.1 to 1.5 μm2 for active AAP cells (Supplementary Table S1).
Abundance and single-cell activity of total and AAP bacteria across environmental gradients
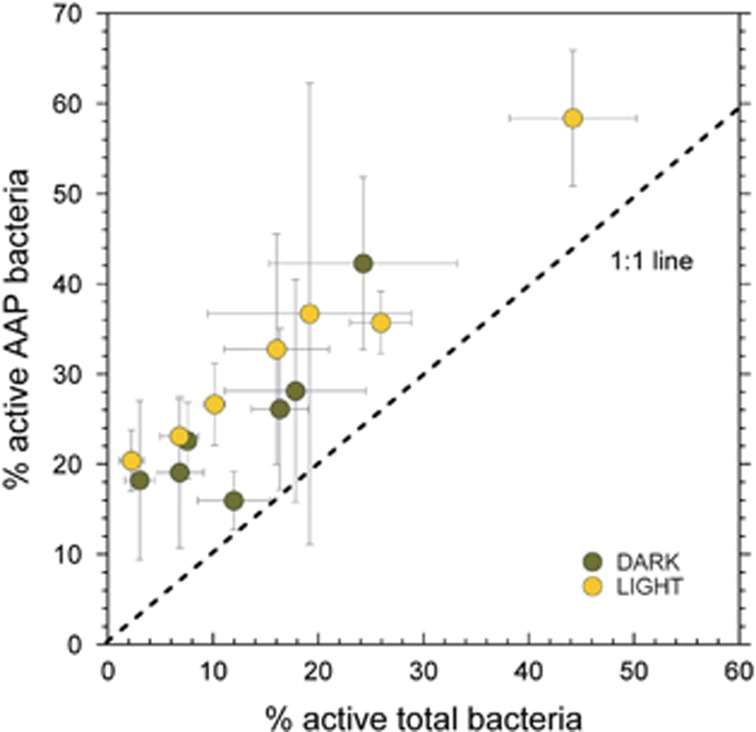
We explored the patterns in abundance, proportion of active cells and the level of cell-specific activity of AAP and total bacteria in relation to the environmental variables listed in Table 2. In general, total and AAP bacterial abundances responded similarly to changes in the measured environmental variables. The abundance of both groups increased with system productivity, being positively related with phosphorous, nitrogen and Chl a concentration (Table 2). The concentration of BChl a was also positively correlated with phosphorous, nitrogen and Chl a concentrations, as well as light attenuation (Table 2). In contrast, the percentage of active cells in both groups decreased with increasing nitrogen, Chl a, DOC and KPAR (Table 2), and there was a strong positive relationship between the proportion of total and AAP active cells (Figure 2). The volume-normalized SGA of AAP and total bacteria were also negatively correlated with Chl a, nitrogen and phosphorous concentrations and, interestingly, the BChl a content per cell was positively correlated with the SGA of AAP cells (Table 2).
Table 2. Correlations between AAP and total bacterial activity or abundance and environmental parameters.
|
Abundance |
% AAP bacteria |
% Active bacteria |
Normalized SGA |
BChl a | Sp BChl a | ||||
|---|---|---|---|---|---|---|---|---|---|
| AAP | TOT | AAP | AAP | TOT | AAP | TOT | AAP | AAP | |
| KPAR | 0.33 | 0.26 | 0.31 | −0.38 | 0.43 | −0.41 | |||
| DN | 0.44 | 0.47 | 0.13 | −0.35 | −0.3 | −0.27 | −0.48 | 0.45 | −0.58 |
| TN | 0.45 | 0.46 | 0.17 | −0.31 | −0.24 | −0.27 | −0.46 | 0.38 | −0.67 |
| DP | 0.45 | 0.53 | −0.14 | −0.36 | −0.48 | 0.24 | −0.77 | ||
| TP | 0.16 | 0.26 | −0.36 | −0.3 | −0.74 | ||||
| Chl a | 0.47 | 0.57 | −0.25 | −0.23 | −0.27 | −0.33 | 0.91 | ||
| DOC | 0.24 | 0.16 | 0.31 | −0.28 | −0.11 | −0.11 | −0.58 | ||
| DOC/Chl a | −0.45 | −0.66 | 0.18 | 0.38 | 0.45 | −0.68 | 0.22 | ||
| Sp BChl a | −0.33 | 0.43 | |||||||
Abbreviations: AAP, aerobic anoxygenic phototrophic bacteria; BChl a, bacteriochlorophyll a; Chl a, chlorophyll a; DN, dissolved nitrogen; DOC, dissolved organic carbon; DP, dissolved phosphorous; KPAR, attenuation coefficient of the photosynthetically active radiation; SGA, silver grain area; Sp BChl a, specific bacteriochlorophyll a; TN, total nitrogen; TOT, total bacteria; TP, total phosphorous.
Pearson's correlation coefficient is shown for significant correlations (α=0.05). Abundance was measured as cells per ml, normalized silver grain area (SGA) as μm2 μm−3, BChl a as ng l−1 and specific BChl a as fg per cell.
Figure 2.
Comparison between the percentage of active AAP cells versus the percentage of active cells in the bulk bacterial community across all lakes. Data from the light and dark incubations for each lake are included for a total of 14 observations. The dashed line indicates a 1:1 relationship between AAP bacteria and the total community. Error bars represent 1 s.d.
Light effects on AAP and total bacterial activity
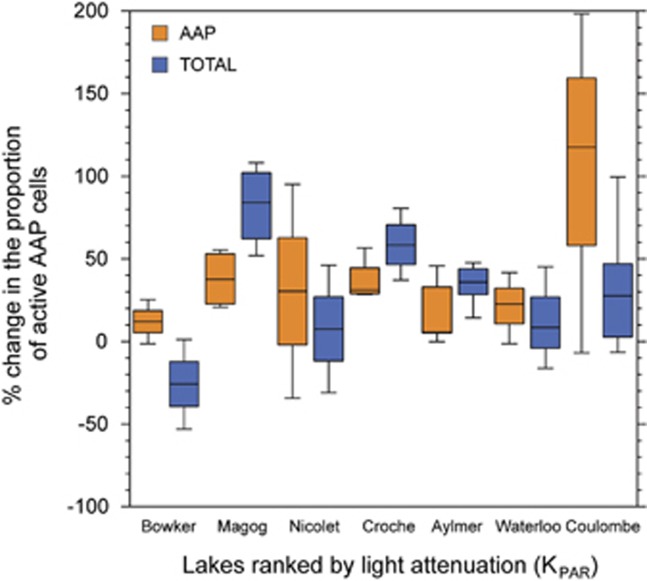
Although AAP and total bacteria behaved similarly with respect to nutrient status, they differed in their responses to light. Overall, the exposure of bacterial communities to light significantly increased the percentage of active cells (P<0.05), but this increase was on average larger for AAP bacteria than for the bulk community; the mean enhancement of the relative number of active cells was 28% versus 37% for total and AAP bacteria, respectively. This enhancement varied greatly among lakes (Figure 3), with the largest enhancement occurring in the lake with the highest light attenuation coefficient. The light-driven stimulation of the proportion of active cells in the bulk community was lower than that of AAP in four out of seven lakes (Figure 3), and the highest stimulation was found in Lake Magog, and this was the only case where an increase in the number of total bacteria was detected after incubation in light conditions. AAP abundance never changed during light incubations when compared with dark-incubated samples or with killed controls (data not shown).
Figure 3.
The response of AAP and total bacteria to exposure to photosynthetically active radiation (PAR) as determined by changes in the proportion of active cells for each of the seven lakes ranked by increasing light attenuation coefficients (KPAR). The light-driven change in the percentage of active cells was calculated as (% activeLight−% activeDark)/% activeDark. The central line indicates the median value of the three replicates, boxes indicate the lower and upper quartiles and whiskers depict the 10th and 90th percentiles.
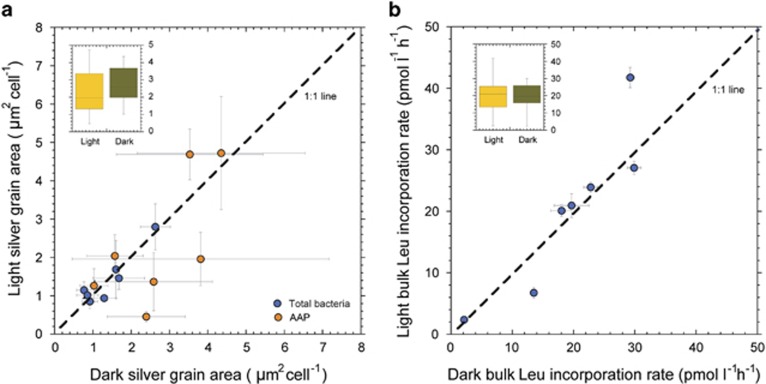
In contrast to the effect on percent active, light had no significant effect on SGA for either active AAP bacteria or active cells in the bulk community, relative to the dark treatment (Figure 4a). Accordingly, there was no effect of light on bulk 3H-leucine assimilation rates (Figure 4b).
Figure 4.
Comparison of the activity of AAP bacteria and of the total bacterial community in light versus dark incubations. Average SGA associated with both active AAP cells and with active cells in the bulk community in light versus dark incubations (a), and bulk 3H-leucine incorporation in light versus dark incubations (b). The dashed line indicates the 1:1 ratio. Error bars are 1 s.d. Insets in each panel show SGA values around active AAP (a) and bulk 3H-leucine incorporation rates (b) between light and dark conditions pooling the seven lakes together.
Contribution of AAP bacteria to total bacterial biomass production
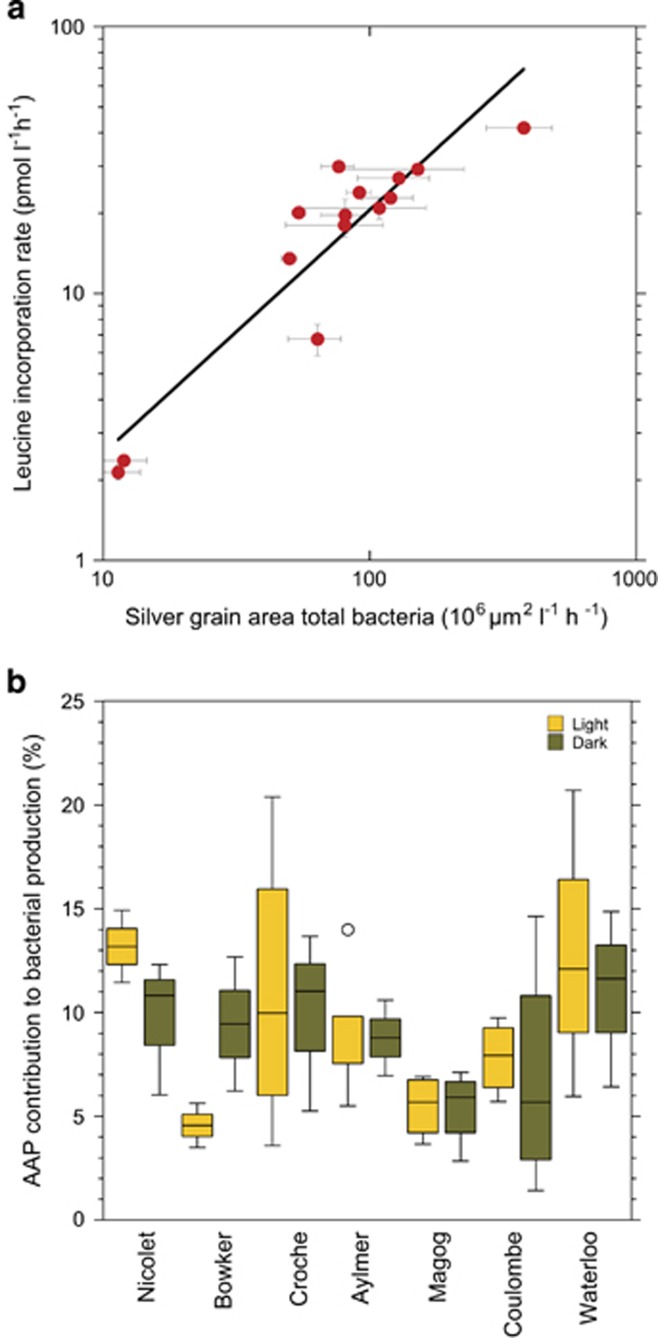
The total SGA of all cells that took up leucine is strongly positively correlated with the corresponding bulk leucine incorporation rates as shown by Sintes and Herndl (2006) in the North Atlantic Ocean. We found a similar relationship for total bacteria in these lakes (Figure 5a). We used this relationship to estimate the potential contribution of AAP bacteria to total leucine incorporation (%BP) by calculating the ratio of SGA around active AAP cells to the SGA around all active bacterial cells. The contribution of AAP bacteria to total leucine incorporation varied among lakes, ranging from 5% to 13% (Figure 5b). There was no significant difference between light and dark incubations when all the lakes were considered together, although the effect of light differed among lakes (Figure 5b). We found no clear pattern in the contribution of AAP bacteria to total bacterial production along gradients of DOC, nutrients or light attenuation.
Figure 5.
Relationship between bulk 3H-leucine incorporation rate and the total average SGA associated with the total bacterial community (a). The numbers are means+s.d. calculated with data from both light and dark incubations for the total community. The solid line is a linear regression fit through all log-transformed data (P<0.05). Contribution of AAP bacteria to total bacterial production in the lakes, ranked by increasing system productivity (b). For each lake, yellow and green bars show the contribution estimated from light and dark incubations, respectively. Central lines indicate median values, boxes indicate the lower and upper quartiles, whiskers depict the 10th and 90th percentiles and the dots represent outliers.
Discussion
The ecological and biogeochemical implications of photoheterotrophic pathways are receiving increasing attention, and during the past decade there have been remarkable advances in our understanding of abundance and distribution patterns of AAP bacteria in lakes and oceans (Hojerová et al., 2011; Ferrera et al., 2013; Fauteux et al., 2015). One of the main challenges in furthering our understanding of the ecological role of this group, and in particular in determining the scenarios where they may have an ecological advantage over their heterotrophic bacterial counterparts, has been determining the actual activity of these bacteria in situ. It is well recognized that aquatic bacterial communities are composed of cells with a wide range of single-cell metabolic activities and physiological states, and that only a fraction of cells within these complex bacterial assemblages are metabolically active at a given time (Del Giorgio and Gasol, 2008). This likely applies to AAP bacteria as well, but until recently it was technically impossible to determine the activity of AAP bacterial cells within bacterial assemblages. The recent development of the AAP-MAR method (Stegman et al., 2014) opened the way to exploring the in situ patterns of activity of AAP bacteria. We applied this approach to seven different lakes in Quebec, representing the first assessment of single-cell activity of freshwater AAP and their contribution to total biomass production in inland waters.
We chose leucine because it has been shown to be taken up by the widest spectrum of freshwater bacteria (see Salcher et al., 2013), and because it is the substrate most commonly used in aquatic studies for routine estimates of bulk bacterial production, therefore allowing for comparisons with previous results. The strong relationship we observed between the total SGA surrounding active cells and the bulk leucine uptake suggests that our approach effectively captures at least the fraction of the community that is active in leucine uptake. It is well recognized, however, that it is unlikely that a single method can be used to describe all the facets of bacterial activity (Smith and del Giorgio, 2003), and that the potential effect of light should be explored on other aspects of cell activity, such as respiration or in the incorporation of other substrates.
The fraction of total and AAP bacteria that took up leucine varied widely among the lakes we examined, as has been typically observed before for the total community (see Smith and del Giorgio, 2003). This variability suggests that there is a large heterogeneity in the proportion of cells that are active in substrate uptake among lakes, yet in all cases, the active fraction of AAP bacteria was always higher than the active fraction of the whole community. In addition, the average specific rate of leucine uptake, based on the SGA around individual cells, was almost twofold higher for active AAP bacteria than for the average active bacterial cell in the community. This difference was slightly higher than that of the two previous MAR-AAP studies (1.6- and 1.4-fold, Kirchman et al., 2014; Stegman et al., 2014), and is also in agreement with previous studies showing that AAP bacteria tend to grow faster than the bulk bacterial community across a diverse range in aquatic habitats (Koblízek et al., 2007; Liu et al., 2010; Ferrera et al., 2011). We have further shown that AAP bacteria were on average larger than the average cell size in the community, a pattern that has been reported before (Sieracki et al., 2006; Lamy et al., 2011; Fauteux et al., 2015). All this evidence indicates that at least a fraction of the AAP bacteria may be an intrinsically fast-growing component of aquatic bacterial communities. However, the large variability in these AAP activity patterns reported here and in the two previous studies (Kirchman et al., 2014; Stegman et al., 2014) suggests that AAP bacteria are far from being a homogeneous functional guild in terms of cell size, single-cell metabolic activity and physiological status.
It is interesting to note that the variation among lakes in the proportion of metabolically active AAP cells was opposite to that in AAP cell abundance: whereas total AAP bacterial abundance tended to increase with lake trophic status, a pattern that has been previously reported (Hojerová et al., 2011; Fauteux et al., 2015), the proportion of active AAP cells and their single-cell activity tended to decline along this same gradient. A similar trend was observed by Stegman et al. (2014) in the Delaware estuary, where the relative abundance of AAP bacteria was highest in the highly productive brackish waters and decreased toward less productive marine waters, but the opposite was true for the proportion of active AAP bacteria. This pattern suggests that the abundance of AAP cells in a system is not a simple function of their growth rate, but also depends on the factors that regulate the loss and the persistence of the active and inactive pool of AAP cells. Regardless of the mechanism, our results indicate that the abundance and activity patterns of AAP bacteria are only loosely coupled, and that conclusions about the ecological role of these prokaryotes cannot be based on abundance patterns alone.
The covariation between AAP and total bacterial abundance observed in previous studies (Salka et al., 2008; Hojerová et al., 2011; Fauteux et al., 2015) suggests that AAP bacteria respond to the same basic environmental drivers and are subject to a similar overall regulation as the bulk bacterial community. Our results support this hypothesis, as we show here that the abundance of active AAP bacteria tracked that of the total bacteria community across lakes. AAP cells appear, however, to be more dynamic than the community as a whole, which was evidenced by a larger variability in the cell size, in the level of single-cell activity, and in the proportion of active cells, relative to the total community. This higher variability in size and activity, which has also been reported in previous studies (Kirchman et al., 2014; Stegman et al., 2014; Fauteux et al., 2015), suggests that although the physiological structure of AAP bacteria and of the bulk community may be influenced by the same overall drivers, the response of AAP bacteria to variations in these drivers may be different and more dynamic than of other heterotrophic bacteria.
In particular, sunlight would be the most likely environmental factor differentially influencing the physiological structure of the AAP bacterial assemblage relative to the bulk bacterial community. In support of this idea, and in accordance to recent results (Stegman et al., 2014), we observed that the percentage of active AAP, but not that of the bulk bacteria, was negatively correlated to light attenuation, suggesting that the proportion of active AAP cells may be enhanced by light availability. We experimentally confirmed this pattern in our light/dark incubations, where we found a light-driven enhancement in the proportion of active AAP cells that was different to the light-driven responses in the bulk bacteria. This enhancement, however, was not accompanied by increases in the average SGA around active AAP cells. The observed light-driven increase in the proportion of active AAP cells could have been due simply to a light-enhanced division rate of active AAP cells, but the abundance of AAP cells did not increase after exposure to light compared with dark incubations or the killed control in any of the lakes (results not shown). These results suggest that light may play a role in the activation of AAP cells, regulating the passage of cells from dormant or slow-growing states to an active-growing state, but without necessarily enhancing the activity rates of individual cells. The hypothesis that light may play a role in the activation of AAP bacteria may in part explain why BChl a-containing AAP cells are found under the ice in northern lakes and in the Arctic Ocean in winter (Cottrell and Kirchman, 2009; Fauteux et al., 2015). As the pigment cannot offer a metabolic advantage when light intensities are near zero, it may facilitate activation of AAP cells when light increases and other environmental conditions become more favorable in the spring.
We found a positive relationship between BChl a per AAP cell and activity per AAP cell as measured by SGA, suggesting that AAP cells may be more active because they have more pigment. This result supports the hypothesis that the small enhancement of AAP bacterial activity due to phototrophy can be accumulated and evidenced over the lifetime of an AAP cell (see Kirchman and Hanson, 2013; Kirchman et al., 2014). Thus, it may be possible that light does increase AAP growth rates but that the enhancement is too small to be measurable in a short incubation experiment.
The larger cell sizes, higher activity and growth rates and preferential grazing of AAP bacteria have repeatedly led to the hypothesis that this group plays a disproportionately large role in the cycling of carbon and nutrients in aquatic food webs (Hojerová et al., 2011; Kirchman et al., 2014; Koblížek, 2015). So far, however, no study had actually quantified their contribution to total bacterial activity in natural ecosystems. We estimated that the contribution of AAP cells to total bacterial community leucine incorporation averaged 10%, and varied from 5% to 17%. These estimated values of potential contribution of AAP bacteria are lower than those reported by Koblízek et al. (2007) and Hojerová et al. (2011) for marine waters, but those studies were based on growth rates estimated from the diurnal decay in BChl a. Regardless, all the evidence suggests that AAP bacteria contribute disproportionately to overall bacterial community activity in aquatic ecosystems.
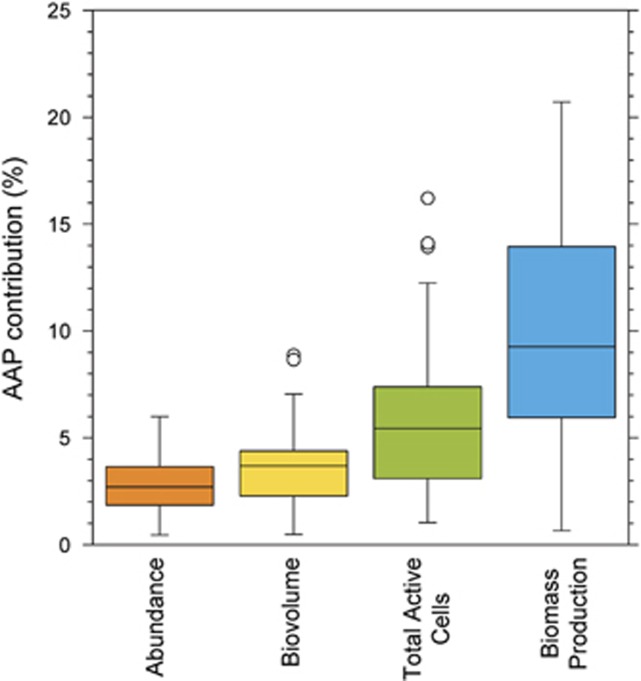
Taken together, our results demonstrate that this ubiquitous group of bacteria represents a very active and dynamic component in freshwater microbial communities. On average, AAP bacteria accounted for 6% of all active cells, and this was twofold higher than their average contribution to total bacterial abundance (Figure 6). As a consequence of their high cell-specific activity and the high relative abundance of active AAP cells, the potential contribution of AAP bacteria to total biomass production was on average twofold higher than their contribution to total bacterial biovolume and threefold higher than their contribution to total bacterial abundance (Figure 6). In addition to light and other environmental properties, grazing and viral lysis not considered here may differentially control the dynamics of the active and inactive pools of AAP cells relative to the rest of heterotrophic bacteria. Indeed, experimental evidence has shown that the larger average cell size and higher cell activity of marine and freshwater AAP seem to render the group particularly vulnerable to grazing (Ferrera et al., 2011; Garcia-Chaves et al., 2015). As there is strong evidence that AAP cells are preferentially grazed even by freshwater crustacean zooplankton (Garcia-Chaves et al., 2015), the contribution of AAP bacteria to the bacterial flow of carbon toward higher trophic levels may be even more important in freshwater ecosystems than in other aquatic habitats.
Figure 6.
Contribution of AAP bacteria to total bacterial abundance, total bacterial biovolume, total active bacterial cells and total bacterial production. The central line indicates the median value calculated with data from both light and dark incubations, the boxes indicate the lower and upper quartiles, the whiskers depict the 10th and 90th percentiles and the circles represent outlier values.
Acknowledgments
We thank AC Mesa, M Morales, K Bareil-Parenteau, A Heathcote and L Yu for field and laboratory assistance, A Parkes, D Vachon and JP Niño for their comments and suggestions on this manuscript and HSC for inspiration. This research was supported by grants from the National Science and Engineering Research Council of Canada (NSERC) to PA del Giorgio.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Biebl H, Wagner-Döbler I. (2006). Growth and bacteriochlorophyll a formation in taxonomically diverse aerobic anoxygenic phototrophic bacteria in chemostat culture: influence of light regimen and starvation. Process Biochem 41: 2153–2159. [Google Scholar]
- Béjà O, Suzuki MT. (2008). Photoheterotrophic marine prokaryotes. In: Kirchman David L (ed). Microbial Ecology of the Oceans. John Wiley & Sons: Hoboken, NJ, USA, pp 131–157. [Google Scholar]
- Cottrell MT, Kirchman DL. (2003). Contribution of major bacterial groups to bacterial biomass production (thymidine and leucine incorporation) in the Delaware estuary. Limnol Oceanogr 48: 168–178. [Google Scholar]
- Cottrell MT, Kirchman DL. (2009). Photoheterotrophic microbes in the Arctic Ocean in summer and winter. Appl Environ Microbiol 75: 4958–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell MTM, Mannino A, Kirchman DL. (2006). Aerobic anoxygenic phototrophic bacteria in the Mid-Atlantic Bight and the North Pacific Gyre. Appl Environ 72: 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giorgio PA, Gasol JM. (2008). Physiological structure and single-cell activity in marine bacterioplankton. In: Kirchman DL (ed). Microbial Ecology of the Oceans. John Wiley & Sons: NJ, USA, pp 243–298. [Google Scholar]
- Fauteux L, Cottrell MT, Kirchman DL, Borrego CM, Garcia-Chaves MC, del Giorgio PA. (2015). Patterns in Abundance, Cell Size and Pigment Content of Aerobic Anoxygenic Phototrophic Bacteria along Environmental Gradients in Northern Lakes. PLoS One 10: e0124035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrera I, Borrego CM, Salazar G, Gasol JM. (2013). Marked seasonality of aerobic anoxygenic phototrophic bacteria in the coastal NW Mediterranean Sea as revealed by cell abundance, pigment concentration and pyrosequencing of pufM gene. Environ Microbiol 16: 2953–2965. [DOI] [PubMed] [Google Scholar]
- Ferrera I, Gasol JM, Sebastián M, Hojerová E, Koblízek M. (2011). Comparison of growth rates of aerobic anoxygenic phototrophic bacteria and other bacterioplankton groups in coastal Mediterranean waters. Appl Environ Microbiol 77: 7451–7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Chaves M, Cottrell M, Kirchman D, Derry A, Bogard M, del Giorgio PA. (2015). Major contribution of both zooplankton and protists to the top-down regulation of freshwater aerobic anoxygenic phototrophic bacteria. Aquat Microb Ecol 76: 71–83. [Google Scholar]
- Hauruseu D, Koblížek M. (2012). The influence of light on carbon utilization in aerobic anoxygenic phototrophs. Appl Environ Microbiol 78: 7414–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojerová E, Mašín M, Brunet C, Ferrera I, Gasol JM, Koblížek M. (2011). Distribution and growth of aerobic anoxygenic phototrophs in the Mediterranean Sea. Environ Microbiol 13: 2717–2725. [DOI] [PubMed] [Google Scholar]
- Kirchman D, Stegman M, Nikrad M, Cottrell MT. (2014). Abundance, size, and activity of aerobic anoxygenic phototrophic bacteria in coastal waters of the West Antarctic Peninsula. Aquat Microb Ecol 73: 41–49. [Google Scholar]
- Kirchman DL, Hanson TE. (2013). Bioenergetics of photoheterotrophic bacteria in the oceans. Environ Microbiol Rep 5: 188–199. [DOI] [PubMed] [Google Scholar]
- Koblízek M, Masín M, Ras J, Poulton AJ, Prásil O. (2007). Rapid growth rates of aerobic anoxygenic phototrophs in the ocean. Environ Microbiol 9: 2401–2406. [DOI] [PubMed] [Google Scholar]
- Koblízek M, Stoń-Egiert J, Sagan S, Kolber ZS. (2005). Diel changes in bacteriochlorophyll a concentration suggest rapid bacterioplankton cycling in the Baltic Sea. FEMS Microbiol Ecol 51: 353–361. [DOI] [PubMed] [Google Scholar]
- Koblížek M. (2015). Ecology of aerobic anoxygenic phototrophs in aquatic environments. FEMS Microbiol Rev 39: 854–870. [DOI] [PubMed] [Google Scholar]
- Lamy D, Jeanthon C, Cottrell MT, Kirchman DL, Van Wambeke F, Ras J et al. (2011). Ecology of aerobic anoxygenic phototrophic bacteria along an oligotrophic gradient in the Mediterranean Sea. Biogeosciences 8: 973–985. [Google Scholar]
- Liu R, Zhang Y, Jiao N. (2010). Diel variations in frequency of dividing cells and abundance of aerobic anoxygenic phototrophic bacteria in a coral reef system of the South China Sea. Aquat Microb Ecol 58: 303–310. [Google Scholar]
- Moran MA, Miller WL. (2007). Resourceful heterotrophs make the most of light in the coastal ocean. Nat Rev Microbiol 5: 792–800. [DOI] [PubMed] [Google Scholar]
- Salcher MM, Posch T, Pernthaler J. (2013). In situ substrate preferences of abundant bacterioplankton populations in a prealpine freshwater lake. ISME J 7: 896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salka I, Moulisová V, Koblízek M, Jost G, Jürgens K, Labrenz M et al. (2008). Abundance, depth distribution, and composition of aerobic bacteriochlorophyll a-producing bacteria in four basins of the central Baltic Sea. Appl Environ Microbiol 74: 4398–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieracki M, Gilg I, Thier E, Poulton NJ, Goericke R. (2006). Distribution of planktonic aerobic anoxygenic photoheterotrophic bacteria in the northwest Atlantic. Limnol Oceanogr 51: 38–46. [Google Scholar]
- Sieracki M, Viles C, Webb K. (1989). Algorithm to estimate cell biovolume using image analyzed microscopy. Cytometry 10: 551–557. [DOI] [PubMed] [Google Scholar]
- Sintes E, Herndl GJ. (2006). Quantifying substrate uptake by individual cells of marine bacterioplankton by catalyzed reporter deposition fluorescence in situ hybridization combined with microautoradiography. Appl Environ Microbiol 72: 7022–7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DC, Azam F. (1992). A simple, economical method for measuring bacterial protein synthesis rates in seawater using 3H-leucine 1. Mar Biol Res 6: 107–114. [Google Scholar]
- Smith E, del Giorgio PA. (2003). Low fractions of active bacteria in natural aquatic communities? Aquat Microb Ecol 31: 203–208. [Google Scholar]
- Spring SS, Lünsdorf HH, Fuchs BMBM Tindall BJBJ. (2009). The photosynthetic apparatus and its regulation in the aerobic gammaproteobacterium Congregibacter litoralis gen. nov., sp. nov. PLoS One 4: e4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegman MR, Cottrell MT, Kirchman DL. (2014). Leucine incorporation by aerobic anoxygenic phototrophic bacteria in the Delaware estuary. ISME J 8: 2339–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkov V, Csotonyi J. (2009). New light on aerobic anoxygenic phototrophs. In: Hunter CN, Daldal F, Thurnauer M, Beatty JT (eds). The Purple Phototrophic Bacteria. Advances in Photosynthesis and Respiration vol. 28 Springer: The Netherlands, pp 31–55. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.