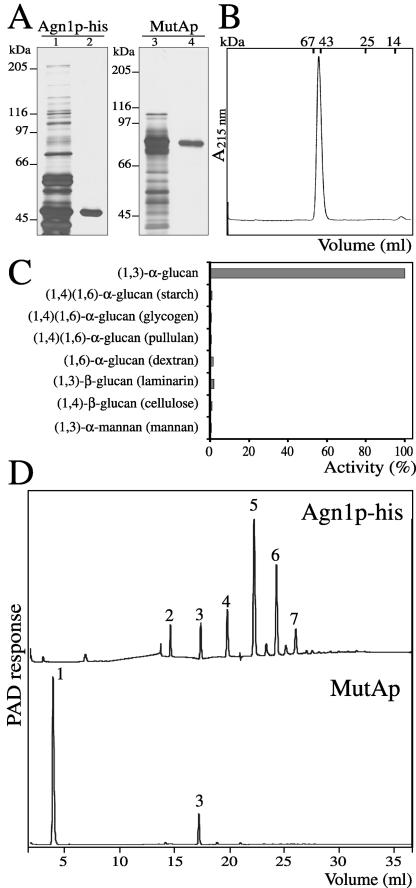
Figure 4.
Agn1p-his is an endo-(1,3)-α-glucanase producing predominantly (1,3)-α-glucan pentasaccharides. (A) Purification of Agn1p-his and T. harzianum MutAp. Agn1p-his was purified from concentrated culture supernatant of strain ND236 by immobilized nickel-nitrilotriacetic acid affinity chromatography (lane 1), followed by anion-exchange chromatography (lane 2). MutAp was purified from a commercial preparation (lane 3) by (1,3)-α-glucan adsorption chromatography (lane 4). Samples were resolved by 8% SDS-PAGE under reducing conditions and visualized by silver staining. (B) Agn1p-his is a monomeric protein. Size-exclusion chromatography of purified Agn1p-his on a Superdex 75 column. Elution volumes of molecular mass markers are indicated. (C) Agn1p-his specifically hydrolyzes (1,3)-α-glucan. Purified Agn1p-his was incubated with indicated substrates at a concentration of 4 mg/ml. To lower backgrounds, substrates were reduced, except for (1,4)-β-glucan (cellulose) and (1,4)(1,6)-α-glucan (starch). To solubilize the (1,3)-α-glucan and (1,4)-β-glucan substrates, they were carboxy-methylated. Data are a percentage of the amount of reducing ends released from reduced and carboxy-methylated (1,3)-α-glucan. (D) Agn1p-his produces mainly (1,3)-α-glucan pentasaccharides. HPAEC analysis with pulsed amperometric detection (PAD) of reaction products released after a 5-h incubation at 37°C from insoluble (1,3)-α-glucan by Agn1p-his (top) or MutAp (bottom). Numbers refer to the degree of polymerization.