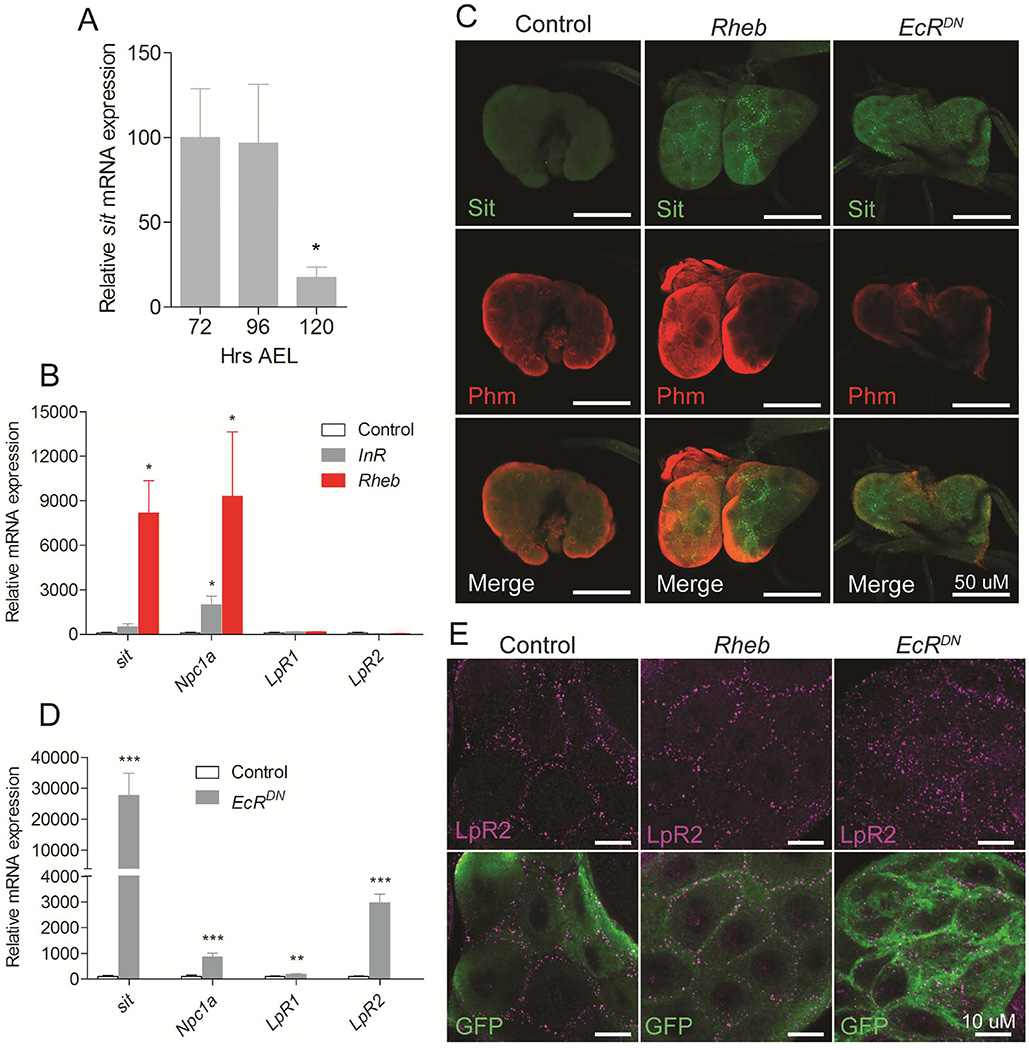
Figure 3. TOR and ecdysone signaling affect cholesterol transport mechanisms in steroid producing tissue.
(A) Expression of sit in the ring gland decreases from the L3 feeding stages (72 and 96 hours AEL) to the late L3 wandering stage (120 hours AEL). (B) Effect on genes involved in cholesterol trafficking in ring glands with activated TOR signaling by overexpression of Rheb or activated insulin signaling by overexpression of the insulin receptor (InR) in the PG. (C) Immunolocalization of Sit using a CRISPR/Cas9 generated knock in of a Venus tag on the endogenous genomic sit locus (Sit-Venus). Detection of the ecdysone biosynthetic enzyme Phantom (Phm) using anti-Phm (red) and Sit-Venus using anti-GFP shows that Sit protein levels (green) increases in the PG cells of the ring glands when Rheb or EcRDN are overexpressed. (D) Effect on genes involved in cholesterol trafficking in ring glands with inhibition of ecdysone feedback regulation by overexpression of a dominant negative form of EcR (EcRDN) in the PG. (E) LpR2 is localized at the cell membrane in both control PG cells and upon Rheb overexpression, while expression EcRDN results in increased and scattered LpR2 distribution throughout cytosol. Detailed description of genotypes is given in Supplemental Experimental Procedures. Error bars indicate SEM (*P<0.05; **P<0.01; ***P<0.001).