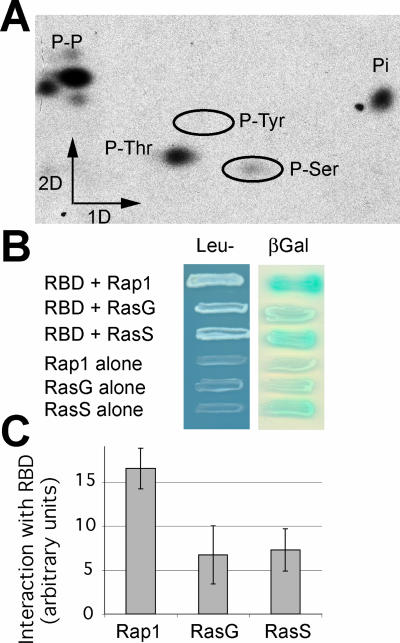
Figure 2.
Characterization of Phg2 kinase activity and ras-binding domain. (A) The Phg2 kinase domain fused to GST was produced in bacteria, partially purified, and used to phosphorylate in vitro a bacterial protein in the presence of [γ-32P]ATP. The phosphorylated protein was purified and hydrolyzed, and the phosphorylated amino acid residues were separated by electrophoresis in the first dimension and ascending chromatography in the second dimension. Phosphorylated serine and threonine residues were detected, as well as unhydrolyzed phosphopeptides (P-P) and Pi (Pi). (B) The ability of the putative Phg2 ras-binding domain to interact with the constitutively active forms of Rap1, RasG, or RasS was tested in a yeast two-hybrid system. Growth in Leu- medium as well as β-galactosidase activity indicated that an interaction took place between the Phg2 RBD and the ras proteins. (C) To evaluate the interaction between the Phg2 RBD and the ras proteins in a more accurate manner, the β-galactosidase activity was measured (e.g., Rap1 + Phg2 RBD), and the background (e.g., Rap1 alone) was subtracted. The average and SEM of four independent experiments are indicated. The Phg2 RBD interacts with all three ras proteins, and most strongly with Rap1.