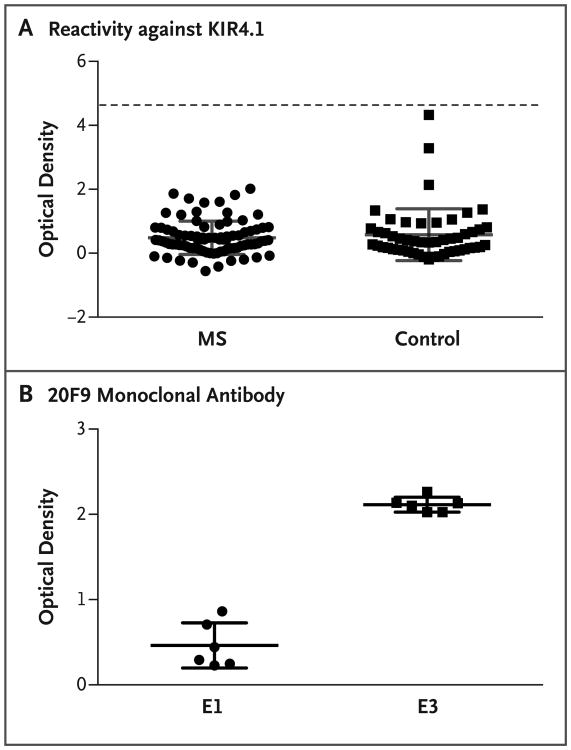
Figure 1. Detection of KIR4.1 Protein in Samples from Patients with Multiple Sclerosis and Controls.
Panel A shows the results of a protein-based enzyme-linked immunosorbent assay (ELISA) that was used to detect serum autoantibodies against KIR4.1 in samples obtained from 86 patients with multiple sclerosis (MS) and 51 healthy controls. The MS samples included serum from 68 patients with relapsing–remitting disease, 8 with secondary progressive disease, 4 with primary progressive disease, and 6 with a clinically isolated syndrome. Purified KIR4.1 tetramers were covalently bound to the plates. The mean (±SD) optical density of the assays was 0.4844±0.5217 in the MS group and 0.5799±0.8107 in the control group (P>0.05 for all comparisons). Each serum sample was tested in duplicate. Positive reactivity (dashed line) was defined as 5 SD above the mean optical density of the control group. Elution 1 (E1) and elution 3 (E3) were obtained by applying increasing concentrations of imidazole and were enriched in high- and low-glycosylated KIR4.1 tetramers, respectively. E1 was therefore used to measure nonspecific binding, and E3, which contains the immunoreactive fraction of KIR4.1, was used to measure the specific autoantibodies. The optical density was measured by dividing the difference between E1 and E3 by E1. Panel B shows the results of an ELISA to evaluate the enrichment of low-glycosylated KIR4.1 tetramers in eluted fractions of histidine-tagged KIR4.1 from a cobalt-chelating column. Two wells of each plate that were used to generate the data in Panel A were incubated with 20F9 monoclonal antibody, which specifically binds to the KIR4.1 tetramers in their low-glycosylation state. The mean optical density was 0.4620±0.2648 for E1 and 2.114±0.08838 for E3 (P = 0.002). Each data point represents the value obtained in a single well. The optical densities in Panels A and B were all corrected for background by subtracting the value obtained at 540 nm from that obtained at 450 nm. The I bars in Panels A and B denote the SD. Statistical analyses were performed with the use of the Mann–Whitney U test.