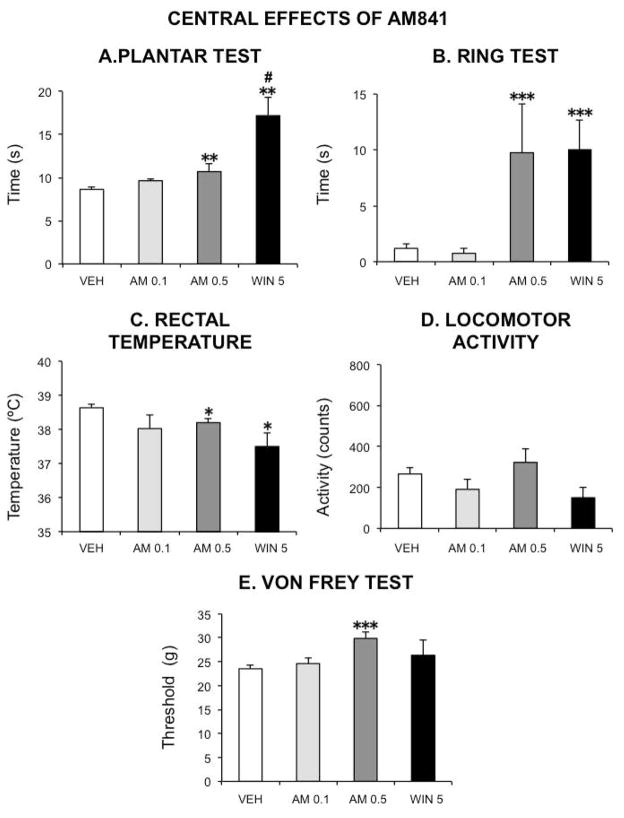
Figure 8.
Central effects of the cannabinoid agonists AM841 and WIN 55,212-2 in the rat. The following tests were used to evaluate the occurrence of the characteristic cannabinoid central effects (cannabinoid tetrad): A–plantar test (for analgesia); Bring test (for catalepsy); C–rectal temperature (for hypothermia); D–spontaneous locomotor activity (for hypolocomotion). The threshold for paw withdrawal in the von Frey test was also measured (E). Vehicle (30 μl kg−1 of Tocrisolve® in saline solution), AM841 (AM, 0.1 or 0.5 mg kg−1) or WIN 55,212-2 (WIN 5, 5 mg kg−1) were intraperitoneally injected. Tests were performed as described in the text. Bars show mean values ± SEM. N ≥ 6 each group. *p <0.05, **p <0.01, ***p<0.001 vs saline (one-way ANOVA followed by Bonferroni post-hoc test). # p<0.05 vs AM 0.5 (unpaired Student’s t-test).