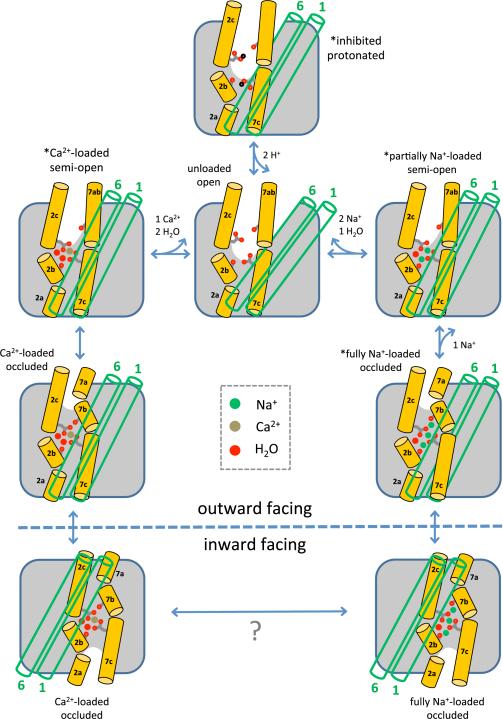
Figure 7.
Structural mechanism of extracellular forward ion exchange in NCX. The carbonyl groups of Ala47 (on TM2b) and Ala206 (on TM7b), and the side chains of Glu54 (on TM2c) and Glu213 (on TM7c) are highlighted; these are four of the key residues for ion chelation and conformational changes. The green open cylinders represent the gating helices TM1 and TM6. Asterisks mark the states whose crystal structures have been determined in this study. These states and their connectivity can also be deduced from the calculated free-energy landscapes, which also reveal a Ca2+-loaded outward-facing occluded state, and an unloaded, fully open state.