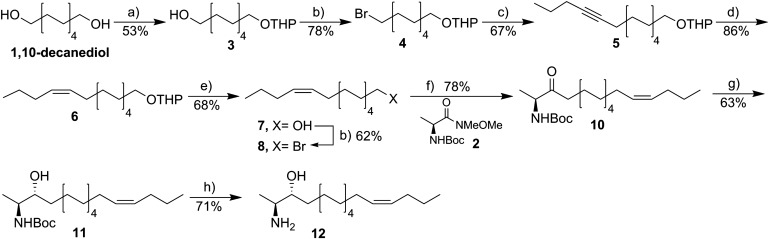
Scheme 1.
Synthesis of the 14Z-deoxySO analog. Reagents and conditions: DHP, p-toluenesulfonic acid (cat.), THF:DCM, 0°C-rt, 12 h (a); CBr4, PPh3, DCM, 0°C-rt, 4 h (b); i) 1-pentyne, tert-BuLi, toluene, −78°C, 1 h, and then ii) DMPU, 4, THF, −78°C-rt, 7 h (c); H2, EDA, Lindlar catalyst, 5% DMF in EtOAc, 0°C, 6 h (d); PPTS (cat.), ethanol, 62°C, 2 h (e); i) Mg, 1,2-DBE (drops), Et2O, reflux, 2 h, and then ii) 2, MeMgBr, DCM:Et2O, reflux, 2 h (f); tri-(tert-butoxy)-aluminum hydride, ethanol, −78°C, 1 h (g); 4 M HCl-dioxane, 0°C, 3 h (h).