Abstract
Study Objectives:
Children with craniofacial anomalies are a heterogeneous group at high risk for obstructive sleep apnea (OSA). However, the prevalence and structural predictors of OSA in this population are unknown. We hypothesized that infants with micrognathia would have more significant OSA than those with isolated cleft palate ± cleft lip (ICP), and those with ICP would have more significant OSA than controls. We postulated that OSA severity would correlate with reduced mandibular size, neurodevelopmental scores, and growth.
Methods:
Prospective cohort study. 15 infants with ICP, 19 with micrognathia, and 9 controls were recruited for polysomnograms, neurodevelopmental testing, cephalometrics (ICP and micrognathia groups) at baseline and a follow-up at 6 mo.
Results:
Baseline apnea-hypopnea index (AHI) [median (range)] of the micrognathia group [20.1 events/h (0.8, 54.7)] was greater than ICP [3.2 (0.3, 30.7)] or controls [3.1 (0.5, 23.3)] (p = 0.001). Polysomnographic findings were similar between ICP and controls. Controls had a greater AHI than previously reported in the literature. Cephalometric measures of both midface hypoplasia and micrognathia correlated with OSA severity. Neurodevelopment was similar among groups. OSA improved with growth in participants with ICP and postoperatively in infants with micrognathia.
Conclusions:
Micrognathia, but not ICP, was associated with more significant OSA compared to controls. Both midface and mandibular hypoplasia contribute to OSA in these populations. OSA improved after surgical correction in most infants with micrognathia, and improved without intervention before palate repair in infants with ICP.
Citation:
Cielo CM, Taylor JA, Vossough A, Radcliffe J, Thomas A, Bradford R, Lioy J, Tapia IE, Assadsangabi R, Shults J, Marcus CL. Evolution of obstructive sleep apnea in infants with cleft palate and micrognathia. J Clin Sleep Med 2016;12(7):979–987.
Keywords: cleft palate, craniofacial, infant, micrognathia, obstructive sleep apnea, pediatrics
INTRODUCTION
Children with craniofacial conditions, such as micrognathia and cleft lip/palate, have been identified as a heterogeneous group at high risk for obstructive sleep apnea (OSA).1 Those with micrognathia are thought to be at risk for obstruction because of the large size of the tongue relative to the other palatal structures.2–4 The mechanism of OSA in children with isolated cleft palate ± cleft lip (ICP) is less clear, but could be due to smaller maxillary and mandibular dimensions.5 Additionally, changes in the upper airway structure could alter upper airway muscle length-tension relationships due to changes in muscle insertion. In some patients with cleft lip, nasal deformities may also contribute to upper airway obstruction. However, studies assessing the risk for OSA in children with ICP6,7 and micrognathia8–10 have largely been retrospective surgical cohorts. There are few prospective data evaluating either of these populations, particularly in younger patients who have not had surgical correction of their palate or mandible.
Children with craniofacial conditions may undergo a variety of corrective surgeries. Mandibular distraction osteogenesis (MDO) is a surgical technique for lengthening the mandible by bilateral corticotomy and then subsequent distraction with either internal or external devices that are then removed as new bone fills the gap. This technique has been used to avoid tracheostomy in children with severe upper airway obstruction and has been shown to be effective in treating OSA in many children with micrognathia,11,12 but has not been studied prospectively.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Children with cleft palate and micrognathia are labeled as high-risk for OSA, but most studies have included patients clinically referred for polysomnography. The predictors of OSA and changes in OSA over the first year of life in these populations are not well understood.
Study Impact: In a group of consecutively-consenting infants, the degree of mandibular and midface hypoplasia but not the presence of cleft palate were associated with more severe OSA. Four months after the baseline evaluation, OSA improved in infants with micrognathia after treatment and those with isolated cleft palate, before palate repair.
The prevalence, consequences, and natural history of OSA in infancy are unknown.13 The effect of OSA on growth and development has also not been well studied. In fact, an infant apnea-hypopnea index (AHI) cutoff for normality has not been well established but recent work has suggested that mean values for 1- and 3-mo old infants are in the range of 1.5 and 0.9 obstructive events per hour, respectively.14 Although older children with OSA usually snore, have observed pauses in breathing, and may exhibit daytime sleepiness or hyperactivity, few infants exhibit these features. Consequently, infant referrals for polysomnography are more likely to come from patients seen as having high-risk diagnoses, such as micrognathia and ICP, than symptoms.15 Considering this, this study was designed to (1) assess differences in OSA in infants with ICP and micrognathia compared with a control group over a 6-mo period, (2) identify cephalometric predictors of OSA, and (3) evaluate neurodevelopmental consequences associated with OSA in this population. We hypothesized that OSA would be more severe in infants with micrognathia compared to those with ICP, and in those with ICP compared to controls. We further hypothesized that OSA severity would correlate with reduced mandibular size and lower neurodevelopmental scores. Additionally, we postulated that surgical correction of micro-gnathia would improve OSA in the group with micrognathia, but that OSA would persist in infants with ICP.
METHODS
Study Design and Participants
This was a single-center prospective cohort study. The families of consecutive infants with ICP or micrognathia who were younger than 6 mo old were approached for participation. Participants were classified as one of three groups: (1) ICP, (2) micrognathia, or (3) healthy infant controls based on the following:
Infants with ICP had a cleft palate and/or cleft lip but did not have any physical examination findings of or a condition causing micrognathia, retrognathia, or glossoptosis, and were recruited from the plastic surgery clinic. Cleft palate was classified using the Veau method by an attending plastic surgeon.16 Class 1 is a cleft of the soft palate alone, class 2 includes clefts of the soft and hard palate, class 3 is a complete unilateral cleft of the lip and palate, and class 4 is a complete bilateral cleft of the lip and palate.
Participants in the micrognathia group had physical examination findings consistent with mandibular hypoplasia, and were recruited from the plastic surgery clinic or from inpatient plastic surgery consultation. Infants in the ICP and micrognathia groups were assessed by an attending physician in the Division of Plastic and Reconstructive Surgery to confirm craniofacial phenotype.
Children in the control group were recruited from the community by advertisements.
For all three groups, infants born at less than 37 w gestation or with a history of intrauterine growth restriction, underlying hypotonia or pulmonary, cardiac, or neurologic conditions were excluded. Participants in the control group had no medical problems and no immediate family history of OSA.
This study was approved by the Children's Hospital of Philadelphia Institutional Review Board. Written informed consent was obtained from parents/guardians of all participants.
Assessments
All participants underwent testing at baseline and at a 6-mo follow-up visit. Testing included physical examination, anthropometrics, overnight polysomnography, and at follow-up assessment, neurodevelopmental testing with the Bayley Scales of Infant and Toddler Development, 3rd edition (BSID-III).17 Participants in the ICP and micrognathia groups had a lateral neck radiograph for cephalometrics. Due to ethical reasons, radiographs were not done in the control group to avoid radiation exposure in otherwise healthy children.
Polysomnography was performed overnight; studies and were conducted and scored according to American Academy of Sleep Medicine pediatric specifications.18 Data were recorded using a Rembrandt polysomnography system (Embla, Broomfield, CO) that included: electroencephalograms (F3-M2, F4-M1, C3-M2, C4-M1, O1-M2, O2-M1), bilateral electrooculograms, chest and abdominal wall motion using respiratory inductance plethysmography, heart rate by electrocardiogram, arterial oxygen saturation (SpO2) by pulse oximetry (Masimo, Irvine, CA); end-tidal PCO2 (PETCO2), measured at the nose by infrared capnometry (Novametrix Medical System, Inc., Wallingford, CT), airflow using a three-pronged thermistor (Pro-Tech Services, Inc., Mukilteo, WA), nasal pressure by a pressure transducer (Pro-Tech Services, Inc., Walnut Cove, NC), and bilateral tibialis anterior electromyogram. Subjects were continuously observed by a polysomnography technician, and were recorded on video with an infrared video camera. Sleep staging was completed using the criteria outlined by Anders for infants during the baseline visit19 and the American Academy of Sleep Medicine criteria for the follow-up visit 6 mo later.20
The BSID-III is a battery of tests and parental-completed questions assessing five domains of early childhood development, including Cognitive, Motor, Language, Adaptive Behavior, and Social-emotional domains.17 The BSID-III has been validated in clinically stable infants from 1 to 42 mo of age. BSID-III testing was performed for all participants by the same psychometrician under the supervision of a licensed psychologist. Composite scores are standardized to have reference values with a mean of 100 and a standard deviation of 15 and are adjusted for age.
Cephalometrics were obtained with infants lying supine with their head rotated 90°. Mandibular length (gonion-gnathion) was the primary outcome measured. Other lengths assessing the midface and mandible included the spheno-occipital synchondrosis-gonion, sella turcica-nasion, sella turcica-maxilla, maxilla-nasion, and sella turcica-C2 (Figure 1). All cephalometric measures were independently repeated by a second radiologist and the concordance correlation coefficient was calculated for each measurement to confirm the reliability of the measurements.
Figure 1. Cephalometric measures.
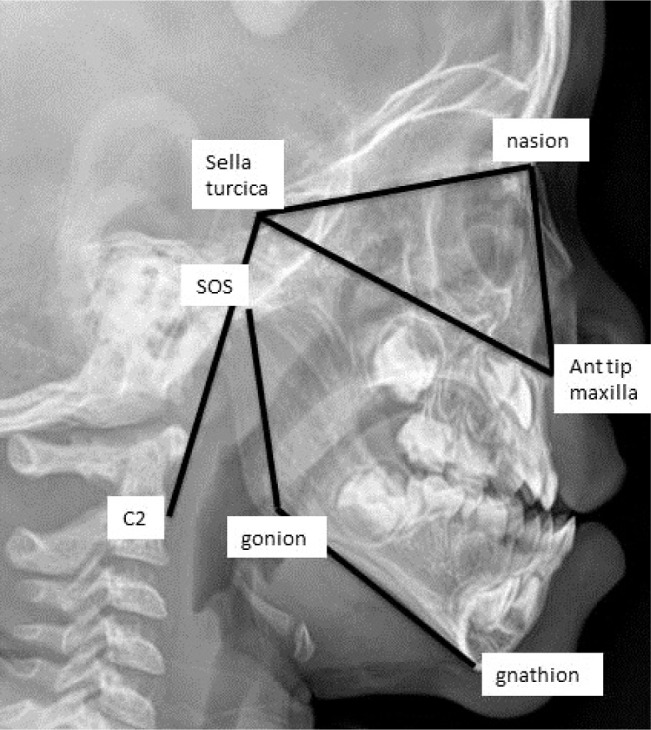
SOS, spheno-occipital synchondrosis.
Feeding information was obtained by standardized clinical assessment by a speech pathologist for groups 1 and 2.
Interventions
Therapeutic medical and surgical decisions (e.g., for cleft repair or MDO) were made based on the patient's clinical condition as part of clinical care. In all participants in the ICP group, cleft lip was repaired after the baseline visit but before the follow-up visit. Participants with persistent OSA after the follow-up polysomnogram were placed on clinical continuous positive airway pressure (CPAP) treatment.
Data Analysis
Data were compared between groups (micrognathia, ICP, and controls) using one-way analysis of variance for normally distributed variables or the nonparametric Kruskal-Wallis oneway analysis by ranks for nonnormally distributed variables (including AHI). AHI normalized after log-transformation, but no differences were seen with repeat analysis using log-transformed AHI and therefore, nonparametric analyses were used. Normality was assessed graphically by constructing histograms and the Shapiro-Wilk test. Two group comparisons (e.g., baseline neurodevelopmental testing between a combined craniofacial group and controls) were made using the two-sample t-test. In addition, linear regression was used to compare groups while also adjusting for age. Baseline and follow-up data were compared within groups using paired t-tests or the nonparametric Wilcoxon signed-rank test. Categorical variables were compared between groups using the chi-square test. Correlations were assessed using Pearson or Spearman rank correlation for data not normally distributed. Statistical analysis was performed using Stata v13.1 (StataCorp College Station, TX) with two-sided tests of hypotheses. The concordance correlation coefficient was performed using R, version 3.1.0 (R Foundation for Statistical Computing, www.R-project.org). A value of p < 0.05 was considered statistically significant.
RESULTS
Demographics
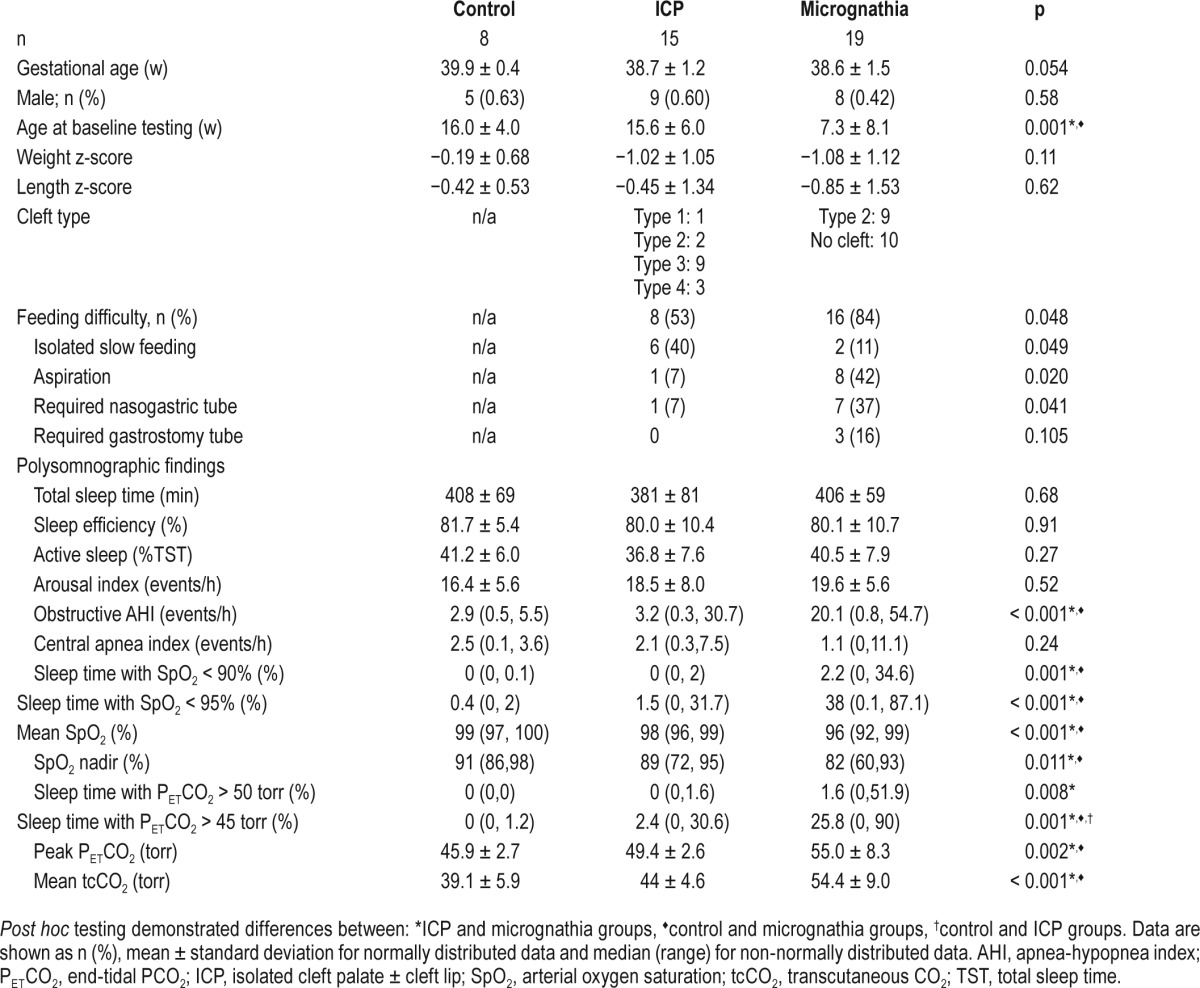
Thirty infants with ICP were approached for participation and 15 declined, either due to time constraints or scheduling conflicts. Twenty infants with micrognathia were approached, and one declined to participate because the family lived far away. In addition, one participant who consented to participate failed to complete baseline testing A total of 33 participants were included in the study, including 19 in the micrognathia group, 15 in the ICP group, and 8 controls (Table 1). Participants in the micrognathia group were significantly younger than those in the ICP and control groups. No subjects had clinically apparent midface hypoplasia.
Table 1.
Baseline demographics and polysomnographic findings.
Baseline Testing
Polysomnograms
Sleep architecture was similar among the three groups (Table 1). On baseline evaluation, obstructive events, oxyhemoglobin saturation, and hypercapnia were worse in the micrognathia group compared to the ICP and control groups. Polysomnographic parameters varied substantially within groups. There was a greater percentage of total sleep time with PETCO2 > 45 torr in the ICP group than the control group, but there were no other significant differences between these two groups.
Physical Examination and Cephalometrics
Physical examination did not reveal enlarged tonsils in any participant. Lateral neck radiograph did not demonstrate any adenoidal hypertrophy. Concordance between the two radiologists evaluating the radiologic studies ranged from 0.89 to 0.94. Infants with micrognathia had reduced mandibular length as well as smaller midface dimensions compared to those with ICP (Table 2). Measures of retrognathia (C2-gnathion and C2-gonion) were not feasible due to patient mouth opening. There was a significant correlation between AHI and measures of overall midface size, midface length, and mandible height (Table 3). However, there was no significant correlation between the AHI and mandibular length or AHI and vertical midface height. When the micrognathia group was analyzed separately, there was not a significant correlation between cephalometric measures and AHI.
Table 2.
Differences in cephalometric measures between the two craniofacial groups.
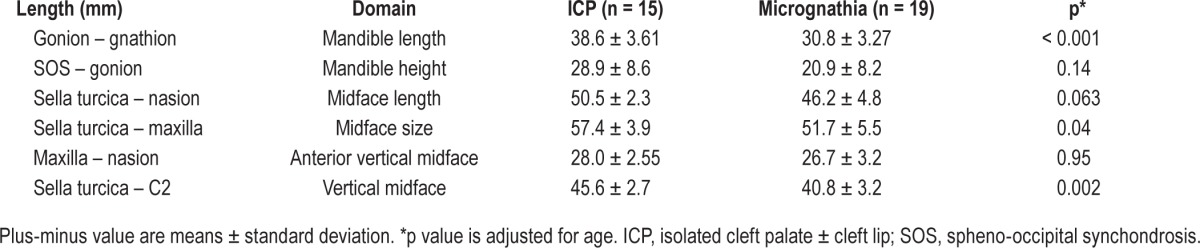
Table 3.
Correlations between of baseline cephalometric measures and apnea-hypopnea index for infants with micrognathia and isolated cleft palate ± cleft lip.

Growth and Feeding
Weight z-scores were lower in the micrognathia and ICP groups compared to controls, but the difference was not statistically significant (p = 0.11, Table 1). Feeding difficulties were more common in infants with micrognathia compared to ICP (Table 1). Specifically, 8 of the 15 (53.3%) participants with ICP and 16 of the 19 (84.2%) with micrognathia had difficulty feeding (p = 0.048). The most common issue was isolated slow feeding, but several subjects were found to have frank aspiration and multiple required feeding tubes for nutrition. Gastroesophageal reflux was clinically diagnosed in nine participants with micrognathia, four with ICP, and one control.
Follow-up Testing
Clinical Follow-up
Of the 19 infants in the micrognathia group, 12 (63%) underwent MDO. One patient was deemed to be a poor candidate for MDO because of small body size, and was treated surgically with tracheostomy. This patient died from an obstructed tracheostomy tube before follow-up testing. All subjects undergoing MDO remained in the neonatal intensive care unit following MDO. Patients remained intubated for 2 to 3 days following surgery and then were extubated, either to room air or CPAP. They remained hospitalized for at least 1 additional week while distraction was initiated and until a feeding plan was established for home. No subjects were discharged with supplemental oxygen. Our center's surgical technique has been described in detail elsewhere.21,22 Of the 15 ICP patients, 10 (67%) also had cleft lip; all cleft lip repairs were completed prior to follow-up testing. For all patients, follow-up testing was complete prior to cleft palate repair. Eleven of the participants with micrognathia (8 of whom had MDO), 11 with ICP, and 7 controls returned for follow-up testing. Baseline AHI of those who returned for testing and those who did not were similar (14.1 ± 15.9 events/h and 15.7 ± 17.2 events/h, respectively, p = 0.77). One patient who had MDO had persistent OSA and was treated with CPAP and two patients with ICP were found to have OSA and were treated with CPAP. All subjects were examined at follow-up testing, and none had enlarged tonsils on physical examination.
Polysomnography
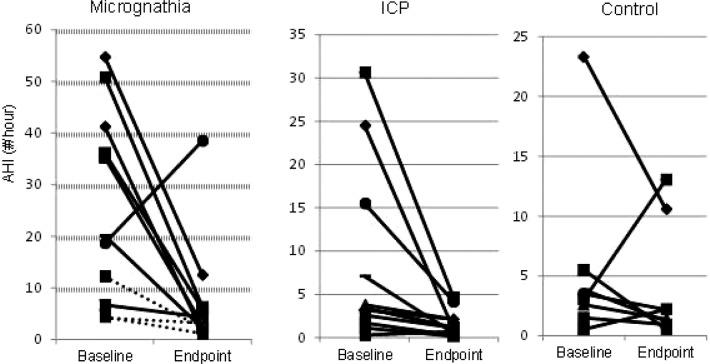
There were significant improvements from baseline to follow-up testing for three of the five variables in the micrognathia group (AHI, sleep time with saturation below 90% and mean transcutaneous CO2) and for the AHI in the ICP group (Table 4). However, two participants with micrognathia had persistently elevated AHI at follow up (Figure 2). All three of the participants with micrognathia who did not have MDO had reduced AHI at the follow-up visit. Although less severe at baseline, there was significant improvement in the AHI of participants in the ICP group as well. There were not significant changes in the polysomnographic parameters in the control group from baseline to follow-up.
Table 4.
Follow-up polysomnography and growth results.
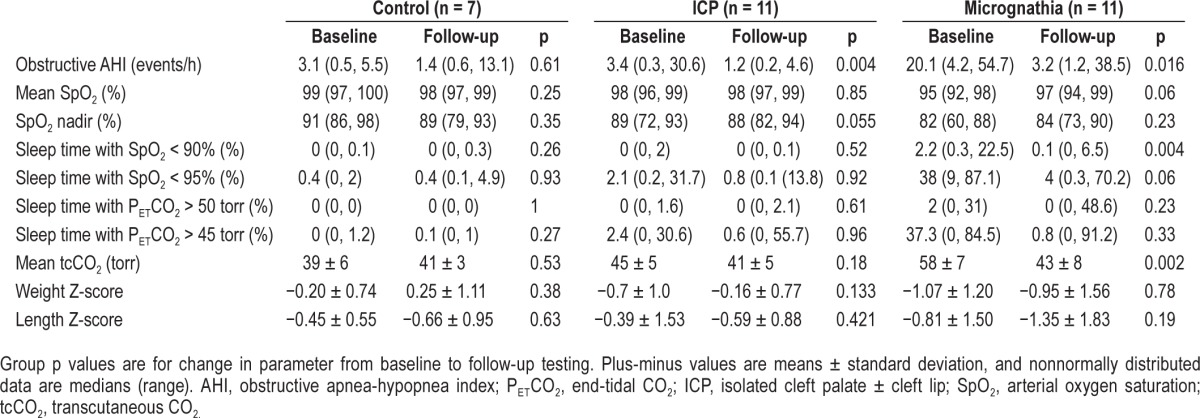
Figure 2. Change in apnea-hypopnea index from baseline to endpoint testing.
For participants with micrognathia, solid lines indicate participants who underwent mandibular distraction, dashed lines indicate those who did not. AHI, apnea-hypopnea index; ICP, isolated cleft palate ± cleft lip.
Cephalometrics
There was similar craniofacial growth between the participants with ICP and those with micrognathia who had MDO. There was no difference in change in cephalometric measures between groups except for mandibular length, for which there was a significantly greater increase in the micrognathia group, reflecting the postsurgical change (Table 5). No subject had enlarged adenoids on the lateral neck radiograph.
Table 5.
Changes in cephalometric measure length from baseline to follow-up testing (n = 11).
Developmental Testing
There was no difference in the composite scores for any of the five domains between the two study groups and the control group (Table 6). There was no significant correlation between baseline AHI and any of the follow-up BSID-III scores.
Table 6.
Composite scores for Bayley Scales of Infant and Toddler Development, 3rd edition on follow-up testing.
Anthropometrics
There was a lower weight z-score in the micrognathia group, but the difference was not statistically significant (Table 4). There was not a significant relationship between AHI and anthropometric measures. For example, there was not a signifi-cant correlation between baseline AHI and the weight z-score at follow-up (Spearman rho = −0.30, p = 0.11).
DISCUSSION
This study demonstrates the evolution of OSA in infants with cleft palate and micrognathia during the first year of life. In addition, to the best of our knowledge, this is the first study to show the evolution of polysomnographic features in a healthy, asymptomatic infant control group. The data here confirmed micrognathia as a more relevant risk factor for OSA than cleft palate status. Importantly, the degree of midface hypoplasia in addition to mandibular hypoplasia correlated with the severity of OSA in this population. OSA improved in the micrognathia group after MDO, and spontaneously in those with ICP, after 6 mo. The AHI in healthy infants was greater than that reported in studies using older methodology.
The current study found no difference in OSA severity in infants with ICP compared to controls, in contrast to other studies. A recent prospective study of infants with cleft lip/ palate found a mean AHI of 7.6 events/h in those with isolated cleft palate and 34.3 events/h in those with Pierre Robin sequence but did not include controls.23 There are several possible explanations for this discrepancy. Our cleft palate cohort included consecutively recruited participants, not a population referred for symptoms of sleep-disordered breathing. The cohort in this study was slightly older than a recently-studied cohort of similar infants,23 which could have resulted in a lower obstructive and central apnea index. The current study also found a greater AHI in control infants than previously described. Importantly, previously published studies have reported polysomnograms in infants performed without nasal pressure, thermistor, and/or electroencephalographic monitoring and older studies did not include hypopneas.14,24 However, there are currently no accepted criteria for defining OSA in infants and the data presented here provide relevant information regarding polysomnographic characteristics of healthy infants. Another important finding in this study was the variability in degree of OSA within each of the three study groups. This could be related to the small sample size in our study or the inherent heterogeneity between infants and children with cranio-facial disorders. Because conditions causing micrognathia are uncommon, we did not limit our analysis to those with a single condition. Larger studies are needed to confirm these findings. There was improvement in the AHI from baseline to follow-up testing in the ICP group, and a trend toward improved AHI in the control group without any intervention. These data suggest improvement in upper airway obstruction in infant populations during the first year of life, but larger studies of infants are needed to confirm these findings.
Because the groups in the current study were relatively small, we chose to analyze cephalometric data for both the ICP and micrognathia groups together. Using this approach, multiple measurements of both midface hypoplasia and micrognathia correlated well with AHI. To our knowledge, this is the first study to correlate cephalometric measures with polysomnography data in children with craniofacial conditions, although MacLean and colleagues used simpler techniques and found that direct facial measurements of the mandibular length correlated with AHI in infants with cleft palate.23 Reduced upper airway caliber is thought to be responsible for the increased risk of OSA in children with craniofacial conditions such as cleft palate or micrognathia. Smaller craniofacial measures and the resultant impact on the airway size have been shown using multiple imaging modalities in this population, although these have not heretofore been correlated with OSA.23 In a study using digital surface photogrammetry to obtain three-dimensional surface imaging, children with Pierre Robin sequence were found to have reduced sagittal midface measurements compared to controls.25 Another study performing cephalometrics in older children found that the midface depth was significantly shorter in those with Pierre Robin sequence than with isolated cleft palate.26 Studies using computed tomography have shown increased length of the mandible27 and increased oropharyngeal airway volume11,28 following correction of micrognathia with MDO in infants. Interestingly, smaller mandibular size is not a feature in otherwise healthy older children with OSA.29 More sophisticated imaging is needed to better understand the relationship between the upper airway structures and OSA in children with craniofacial conditions.
In the current cohort, there was no significant difference in BSID-III scores between the three groups. Children with craniofacial conditions including micrognathia and ICP have been shown to have reduced scores on neurocognitive testing compared with age-matched controls. Two studies evaluating young children with ICP found lower BSID scores compared with controls at ages ranging from 3 to 24 mo.30,31 In contrast, a more recent study of 3-y-olds with cleft lip/palate found difference only in expressive and receptive language.32 Another study evaluating school-age children with Pierre Robin sequence found reduced scores on multiple subsets of the Kaufman-Assessment Battery for Children (sequential processing, simultaneous processing, and achievement) compared with healthy controls, although mean scores for both groups were within the normal range.33 A recent meta-analysis found reduced cognitive functioning in multiple domains in older children and adults with ICP compared with controls.34 There was a trend toward more participants in the ICP and micro-gnathia groups having cognitive scores less than one standard deviation below the published mean. Thus, a larger sample size may have detected true differences between the groups. Additionally, developmental differences that become apparent in older children may not always be readily detected in infants, for whom neurodevelopmental tests are less refined. It is also possible that early detection and treatment of OSA prevents later cognitive declines.
Although the study results showed a trend toward a lower weight z-score in the micrognathia group at follow-up testing, there was not a significant correlation between the AHI and follow-up weight z-score or length z-score. There was significant variability within study groups. One study of 3-y-old children with cleft palate found a negative correlation between AHI and weight z-score.32 As has been shown in other studies,35 feeding difficulties were common in our cohort, especially in infants with micrognathia. Further studies are needed to clarify the relationship between OSA or other factors and growth.
This study had several strengths and several limitations. Strengths included the prospective longitudinal nature of the study, standardized cephalometric evaluation, and the inclusion of a control group. The small sample size was a limitation of this study. Larger prospective studies of healthy infants are needed to better establish normative values in this age group. Larger prospective studies of infants with specific craniofacial conditions are needed to help explain the variability seen in this cohort. In addition, because this cohort was referred from a large catchment area, follow-up testing was not possible in all participants. It could be possible that only participants with more severe OSA would return for follow-up testing, leading to referral bias. However, it is reassuring that the baseline AHI of those who returned for testing and those who did not were similar. Finally, single-view cephalometric imaging provides data that are limited to two dimensions and does not allow for the investigation of airway volumes or three-dimensional analysis of soft tissue and bone, but is easily obtained and does not require sedation.
In conclusion, this study has shown that in infants with micrognathia and midface hypoplasia are significant risk factors for OSA whereas isolated cleft palate does not appear to contribute substantially. There are differences in the craniofacial features between these two groups that extend to the midface, including the distances from the sella turcica to nasion and sella turcica to the anterior tip of the maxilla. These cephalometric data underscore the need for more sophisticated imaging studies in these patients, in addition to polysomnography to better understand the relationship between OSA and specific craniofacial features. Another important finding is that OSA improves with growth in infants with ICP before the cleft palate is repaired. MDO is successful in most cases in infants with micrognathia, but the natural history and long-term surgical success remain unknown in this population.
DISCLOSURE STATEMENT
This was not an industry supported study. Financial Support was from NIH 5T32HL007713-20, UL1RR024134, KL2 TR000139, NIH HL58585. Dr. Vossough has received consultant fees from Banyan and royalties from Oxford University Press, unrelated to this article. Dr. Radcliffe has received consultant fees from the City of Philadelphia, unrelated to this article. Dr. Marcus has received research support from Philips Respironics, unrelated to this article. The other authors have indicated no financial conflicts of interest. There was nothing used off-label in this study. The study was done at The Children's Hospital of Philadelphia, Philadelphia, PA.
ACKNOWLEDGMENTS
The authors are grateful to the infants who participated in this study and their families. Study data were collected and managed using REDCap electronic data capture tools hosted at The Children's Hospital of Philadelphia.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BSID
Bayley Scales of Infant and Toddler Development
- CPAP
continuous positive airway pressure
- ICP
isolated cleft palate ± cleft lip
- MDO
mandibular distraction osteogenesis
- OSA
obstructive sleep apnea
- PETCO2
end-tidal PCO2
- SpO2
arterial oxygen saturation
REFERENCES
- 1.Marcus CL, Brooks LJ, Draper KA, Gozal D. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:1–9. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 2.Bookman LB, Melton KR, Pan BS, et al. Neonates with tongue-based airway obstruction: a systematic review. Otolaryngol Head Neck Surg. 2012;146:8–18. doi: 10.1177/0194599811421598. [DOI] [PubMed] [Google Scholar]
- 3.Daniel M, Bailey S, Walker K, et al. Airway, feeding and growth in infants with Robin sequence and sleep apnoea. Int J Pediatr Otorhinolaryngol. 2013;77:499–503. doi: 10.1016/j.ijporl.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Lidsky ME, Lander TA, Sidman JD. Resolving feeding difficulties with early airway intervention in Pierre Robin Sequence. Laryngoscope. 2008;118:120–3. doi: 10.1097/MLG.0b013e31815667f3. [DOI] [PubMed] [Google Scholar]
- 5.Hermann NV, Kreiborg S, Darvann TA, Jensen BL, Dahl E, Bolund S. Early craniofacial morphology and growth in children with unoperated isolated cleft palate. Cleft Palate Craniofac J. 2002;39:604–22. doi: 10.1597/1545-1569_2002_039_0604_ecmagi_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 6.Muntz H, Wilson M, Park A, Smith M, Grimmer JF. Sleep disordered breathing and obstructive sleep apnea in the cleft population. Laryngoscope. 2008;118:348–53. doi: 10.1097/MLG.0b013e318158195e. [DOI] [PubMed] [Google Scholar]
- 7.MacLean JE, Fitzsimons D, Hayward P, Waters KA, Fitzgerald DA. The identification of children with cleft palate and sleep disordered breathing using a referral system. Pediatr Pulmonol. 2008;43:245–50. doi: 10.1002/ppul.20763. [DOI] [PubMed] [Google Scholar]
- 8.Dykes EH, Raine PA, Arthur DS, Drainer IK, Young DG. Pierre Robin syndrome and pulmonary hypertension. J Pediatr Surg. 1985;20:49–52. doi: 10.1016/s0022-3468(85)80391-2. [DOI] [PubMed] [Google Scholar]
- 9.Cozzi F, Pierro A. Glossoptosis-apnea syndrome in infancy. Pediatrics. 1985;75:836–43. [PubMed] [Google Scholar]
- 10.Anderson IC, Sedaghat AR, McGinley BM, Redett RJ, Boss EF, Ishman SL. Prevalence and severity of obstructive sleep apnea and snoring in infants with Pierre Robin sequence. Cleft Palate Craniofac J. 2011;48:614–8. doi: 10.1597/10-100. [DOI] [PubMed] [Google Scholar]
- 11.Rachmiel A, Emodi O, Aizenbud D. Management of obstructive sleep apnea in pediatric craniofacial anomalies. Ann Maxillofac Surg. 2012;2:111–5. doi: 10.4103/2231-0746.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin SY, Halbower AC, Tunkel DE, Vanderkolk C. Relief of upper airway obstruction with mandibular distraction surgery: long-term quantitative results in young children. Arch Otolaryngol Head Neck Surg. 2006;132:437–41. doi: 10.1001/archotol.132.4.437. [DOI] [PubMed] [Google Scholar]
- 13.Katz ES, Mitchell RB, D'Ambrosio CM. Obstructive sleep apnea in infants. Am J Respir Crit Care Med. 2012;185:805–16. doi: 10.1164/rccm.201108-1455CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brockmann PE, Poets A, Poets CF. Reference values for respiratory events in overnight polygraphy from infants aged 1 and 3months. Sleep Med. 2013;14:1323–7. doi: 10.1016/j.sleep.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Ramgopal S, Kothare SV, Rana M, Singh K, Khatwa U. Obstructive sleep apnea in infancy: a 7-year experience at a pediatric sleep center. Pediatr Pulmonol. 2014;49:554–60. doi: 10.1002/ppul.22867. [DOI] [PubMed] [Google Scholar]
- 16.Kirschner RE, LaRossa D. Cleft lip and palate. Otolaryngol Clin North Am. 2000;33:1191–215. v–vi. doi: 10.1016/s0030-6665(05)70277-2. [DOI] [PubMed] [Google Scholar]
- 17.Bayley N. San Antonio, TX: Harcourt Assessment Inc; 2006. Bayley scales of infant and toddler development. [Google Scholar]
- 18.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL, Vaughn BV for the American Academy of Sleep Medicine. Darien, IL: American Academy of Sleep Medicine; 2012. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.0. www.aasmnet.org. [Google Scholar]
- 19.Anders T, Emde R, Parmelee A. Los Angeles, CA: Brain Information Service/Brain Research Institute; 1971. A manual of standardized terminology: Techniques and criteria for scoring states of sleep and wakefulness in newborn infants. [Google Scholar]
- 20.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, 1st ed. [Google Scholar]
- 21.Chung CU, Yu JW, Bastidas N, Bartlett SP, Taylor JA. Utility of the ultrasonic scalpel in mandibular distraction osteogenesis. J Craniofac Surg. 2012;23:1279–82. doi: 10.1097/SCS.0b013e31825196b4. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein JA, Chung C, Paliga JT, et al. Mandibular distraction osteogenesis for the treatment of neonatal tongue-based airway obstruction. J Craniofac Surg. 2015;26:634–41. doi: 10.1097/SCS.0000000000001416. [DOI] [PubMed] [Google Scholar]
- 23.Maclean JE, Fitzsimons D, Fitzgerald DA, Waters KA. The spectrum of sleep-disordered breathing symptoms and respiratory events in infants with cleft lip and/or palate. Arch Dis Child. 2012;97:1058–63. doi: 10.1136/archdischild-2012-302104. [DOI] [PubMed] [Google Scholar]
- 24.Kato I, Franco P, Groswasser J, Kelmanson I, Togari H, Kahn A. Frequency of obstructive and mixed sleep apneas in 1,023 infants. Sleep. 2000;23:487–92. [PubMed] [Google Scholar]
- 25.Krimmel M, Kluba S, Breidt M, et al. Three-dimensional assessment of facial development in children with Pierre Robin sequence. J Craniofac Surg. 2009;20:2055–60. doi: 10.1097/SCS.0b013e3181be87db. [DOI] [PubMed] [Google Scholar]
- 26.Daskalogiannakis J, Ross RB, Tompson BD. The mandibular catch-up growth controversy in Pierre Robin sequence. Am J Orthod Dentofacial Orthop. 2001;120:280–5. doi: 10.1067/mod.2001.115038. [DOI] [PubMed] [Google Scholar]
- 27.Denny AD, Talisman R, Hanson PR, Recinos RF. Mandibular distraction osteogenesis in very young patients to correct airway obstruction. Plast Reconstr Surg. 2001;108:302–11. doi: 10.1097/00006534-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Pfaff MJ, Metzler P, Kim Y, Steinbacher DM. Mandibular volumetric increase following distraction osteogenesis. J Plast Reconstr Aesthet Surg. 2014;67:1209–14. doi: 10.1016/j.bjps.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Schiffman PH, Rubin NK, Dominguez T, et al. Mandibular dimensions in children with obstructive sleep apnea syndrome. Sleep. 2004;27:959–65. doi: 10.1093/sleep/27.5.959. [DOI] [PubMed] [Google Scholar]
- 30.Speltz ML, Endriga MC, Hill S, Maris CL, Jones K, Omnell ML. Cognitive and psychomotor development of infants with orofacial clefts. J Pediatr Psychol. 2000;25:185–90. doi: 10.1093/jpepsy/25.3.185. [DOI] [PubMed] [Google Scholar]
- 31.Jocelyn LJ, Penko MA, Rode HL. Cognition, communication, and hearing in young children with cleft lip and palate and in control children: a longitudinal study. Pediatrics. 1996;97:529–34. [PubMed] [Google Scholar]
- 32.Smith CB, Walker K, Badawi N, Waters KA, MacLean JE. Impact of sleep and breathing in infancy on outcomes at three years of age for children with cleft lip and/or palate. Sleep. 2014;37:919–25. doi: 10.5665/sleep.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drescher FD, Jotzo M, Goelz R, Meyer TD, Bacher M, Poets CF. Cognitive and psychosocial development of children with Pierre Robin sequence. Acta Paediatr. 2008;97:653–6. doi: 10.1111/j.1651-2227.2008.00742.x. [DOI] [PubMed] [Google Scholar]
- 34.Roberts RM, Mathias JL, Wheaton P. Cognitive functioning in children and adults with nonsyndromal cleft lip and/or palate: a meta-analysis. J Pediatr Psychol. 2012;37:786–97. doi: 10.1093/jpepsy/jss052. [DOI] [PubMed] [Google Scholar]
- 35.Reid J, Kilpatrick N, Reilly S. A prospective, longitudinal study of feeding skills in a cohort of babies with cleft conditions. Cleft Palate Craniofac J. 2006;43:702–9. doi: 10.1597/05-172. [DOI] [PubMed] [Google Scholar]