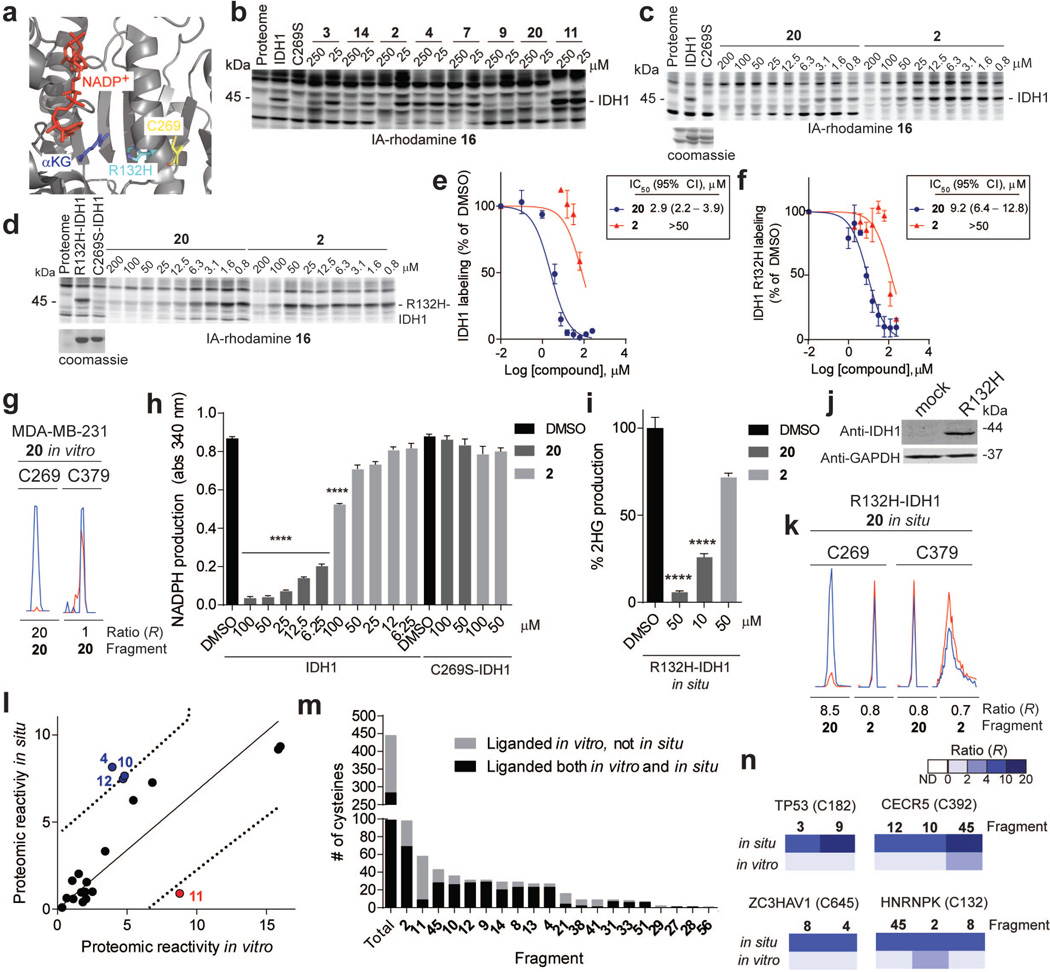
Extended Data Figure 6. IDH1-related and general in situ activity of fragment electrophiles.
a, X-ray crystal structure of IDH1 (PDB ID: 3MAS) showing the position of C269 and the frequently mutated residue in cancer, R132. b, Blockade of 16 labeling of WT-IDH1 by representative fragment electrophiles. Recombinant, purified WT-IDH1 was added to MDA-MB-231 lysates at a final concentration of 2 µM, treated with fragments at the indicated concentrations, followed by IA-rhodamine probe 16 (2 µM) and analysis by SDS-PAGE and in-gel fluorescence scanning. Note that a C269S mutant of IDH1 did not label with IA-rhodamine 16. c, d, Reactivity of 20 and control fragment 2 with recombinant, purified WT-IDH1 (b) or R132H–IDH1 (c) added to MDA-MB-231 lysates to a final concentration of 2 or 4 µM protein, respectively. Fragment reactivity was detected in competition assays using the IA-rhodamine probe (2 µM); note that the C269S–IDH1 mutant did not react with IA-rhodamine. e, f, Apparent IC50 curve for blockade of IA-rhodamine-labeling of IDH1 (e) and R132H–IDH1 (f) by 20. Note that the control fragment 2 showed much lower activity. g, Representative MS1 ion chromatograms for the IDH1 tryptic peptides containing liganded cysteine C269 and an unliganded cysteine C379 quantified by isoTOP-ABPP in MDA-MB-231 lysates treated with fragment 20 (25 µM). h, 20, but not 2, inhibited IDH1-catalyzed oxidation of isocitrate to α-ketoglutarate (α-KG) as measured by an increase in NADPH production (340 nm absorbance). 20 did not inhibit the C269S–IDH1 mutant. i, 20 inhibited oncometabolite 2-hydroxyglutarate (2-HG) production by R132H–IDH1. MUM2C cells stably overexpressing the oncogenic R132H–IDH1 mutant or control GFP-expressing MUM2C cells were treated with the indicated fragments (2 h, in situ). Cells were harvested, lysed and IDH1-dependent production of 2-HG from α-KG and NADPH was measured by LC-MS and from which 2-HG production of GFP-expressing MUM2C cells was subtracted (GFP-expressing MUM2C cells produced < 10% of the 2-HG generated by R132H–IDH1-expressing MUM2C cells). j, Western blot of MUM2C cells stably overexpressing GFP (mock) or R132H–IDH1 proteins. k, Representative MS1 chromatograms for the IDH1 tryptic peptides containing liganded cysteine C269 and an unliganded cysteine C379 quantified by isoTOP-ABPP in R132H–IDH-expressing MUM2C lysates treated with 20 or control fragment 2 (50 µM, 2 h, in situ). l, Proteomic reactivity values for individual fragments are comparable in vitro and in situ. One fragment (11) marked in red showed notably lower reactivity in situ versus in vitro. Reactivity values were calculated as in Fig. 1c. Dashed line mark 90% prediction intervals for the comparison of in vitro and in situ proteomic reactivity values for fragment electrophiles. Blue and red circles mark fragments that fall above (or just at) or below these prediction intervals, respectively. m, Fraction of cysteines liganded in vitro that are also liganded in situ. Shown are liganded cysteine numbers for individual fragments determined in vitro and the fraction of these cysteines that were liganded by the corresponding fragments in situn, Representative cysteines that were selectively targeted by fragments in situ, but not in vitro. For in situ-restricted fragment-cysteine interactions, a second cysteine in the parent protein was detected with an unchanging ratio (R ~ 1), thus controlling for potential fragment-induced changes in protein expression. For panels e, f, h and i, data represent mean values ± SEM for at least three independent experiments. Statistical significance was calculated with unpaired students t-tests comparing DMSO- to fragment-treated samples; ****, p < 0.0001.