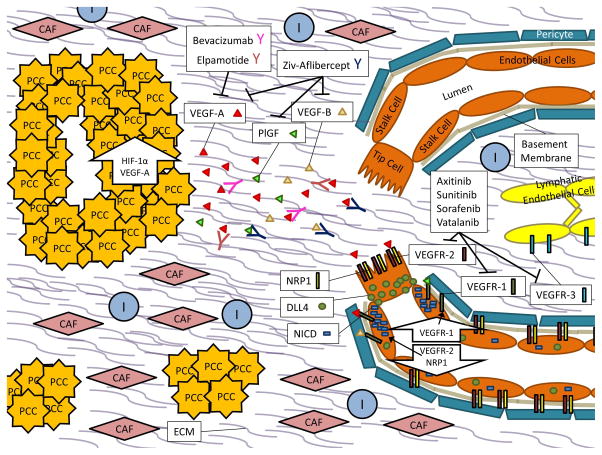
Figure 1. PDAC Angiogenesis.
In PDAC, Pancreatic Cancer Cells (PCCs) proliferate within a desmoplastic stroma that consists of both cellular components such as Cancer Associated Fibroblasts (CAFs), Immune Cells (Is), and Endothelial Cells (ECs) as well as Extracellular Matrix (ECM) components like soluble growth factors, cytokines, collagens, fibronectin, laminin, glycoproteins, and proteoglycans.
Up-regulation of hypoxia inducible factor 1, alpha subunit (gene: HIF1A) (HIF-1α) and the pro-angiogenic molecule VEGF-A within PCCs results in secretion of VEGF-A molecules into the tumor microenvironment. When VEGF-A signals through VEGFR-2 and its NRP1 co-receptor on endothelial cells, downstream signaling results in increased expression of DLL4. DLL4 will bind to Notch receptors on neighboring cells, subsequently releasing NICD, which then down-regulates VEGFR-2 and NRP1 expression and up-regulates expression of the VEGFR-1 decoy receptor. This favors migration of a tip cell towards the VEGF-A gradient while the neighboring stalk cells become de-sensitized to the signal. In the quiescent vasculature, DLL4 and Notch signaling are balanced.
Small molecule inhibitors of angiogenesis, such as Axitinib, Sunitinib, Sorafenib, and Vatalanib primarily act on the vascular endothelial growth factor receptor complexes (VEGFR-1, VEGFR-2, and Vascular endothelial growth factor receptor 3 (gene: FLT4) (VEGFR-3)) while recombinent protein inhibitors of angiogenesis like Bevacizumab, Elpamotide, and Ziv-Aflibercept act on vascular endothelial growth factor ligands like VEGF-A, vascular endothelial growth factor B (gene: VEGFB) (VEGF-B), and/or placenta growth factor (gene: PGF) (PlGF).