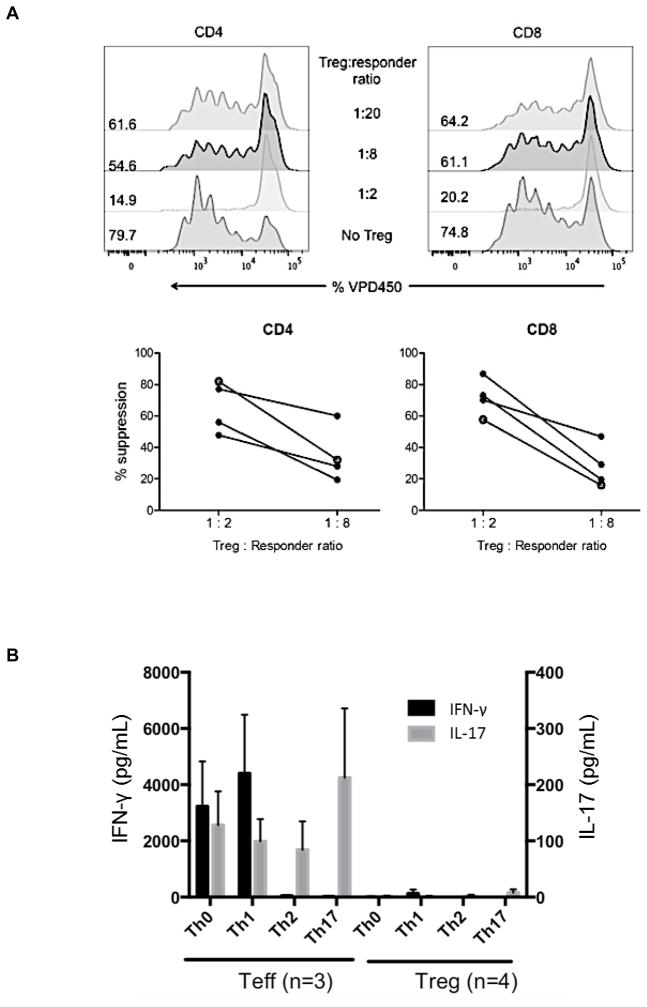
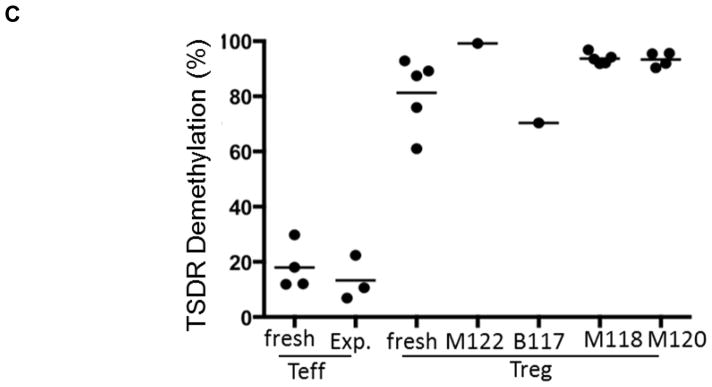
Figure 2. Suppressive function and stability of ex vivo-expanded cynomolgus Treg infused into heart graft recipients.
(A) Ex-vivo-expanded Treg were tested for their ability to suppress VPD450-labeled autologous or allogeneic anti-CD3/CD28-activated CD4+ and CD8+ T cell proliferation. Percent divided cells was calculated using FlowJo software (top). The suppressive effect of this representative batch of Treg was tested on autologous (open circles) and three different allogeneic (closed circles) activated T cells (bottom). Treg function was expressed as percent suppression of T cell proliferation calculated using the formula: (percent divided T cells without addition of Treg – percent divided T cells with Treg)/percent divided T cells without addition of Treg x 100%. The expanded Treg showed strong suppressive activity when added to bead-stimulated T cells. Data are representative of the 4 batches of expanded Treg used for infusion after transplantation. (B) Primary CD4+CD25− effector T cells (Teff) or Treg expanded ex vivo for 3 weeks were stimulated with NHP-specific anti-CD3/CD28 microbeads at a cell:bead ratio of 10:1 for 4 days under the following culture conditions: Th1 (IL-12 + anti-CD4 mAb), Th2 (IL-4 + anti-IFN-γ mAb), or Th17 (IL-6 + IL-21 + IL-23 + IL-1β + TGF-β + anti-IL-4 mAb + anti-IFN-γ mAb). Supernatants were collected and assayed for IFN-γ and IL-17 using CBA kits. Data (means ± 1SD) are from n=3 or n=4 separate individual Teff or Treg cultures. (C) Demethylation status of Foxp3 TSDR in freshly-isolated Treg and Treg expanded for individual or multiple infusions from 4 different monkeys for 3 weeks before infusion into heart allograft recipients. Data are also shown for freshly-isolated and expanded Teff.