FIGURE 2.
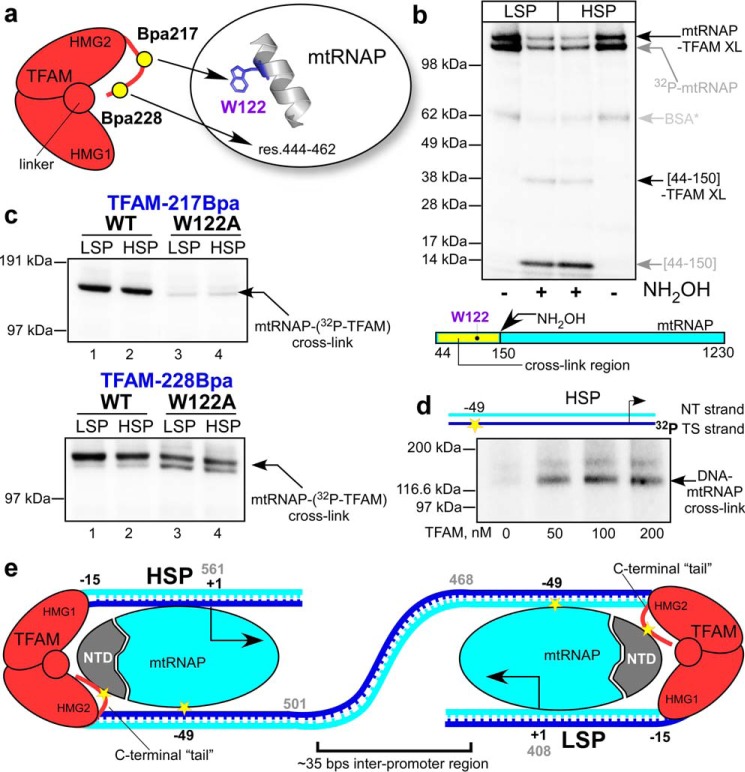
Topology of the pre-initiation complexes assembled on LSP and HSP. a, schematics of TFAM interactions with mtRNAP on LSP. Two major interactions involve TFAM region next to residue 217 and Trp-122 in the N-terminal extension region of mtRNAP (residues (res.) 120–134, predicted α-helix) and TFAM region next to residue 228 and mtRNAP region 444–463. b, the C-terminal region of TFAM interacts with the N-terminal extension region of mtRNAP in the pre-ICs assembled on LSP or HSP promoter. The pre-ICs were UV-irradiated, treated with hydroxylamine (NH2OH), and resolved using SDS-PAGE. The radioactive species (∼37 kDa) that appear upon the treatment of the pre-IC with hydroxylamine represent TFAM cross-link to the region 44–150 of mtRNAP. XL, cross-link. c, MtRNAP residue Trp-122 is a primary interaction target for TFAM in complexes assembled on both promoters. Upper panel, cross-linking performed using 32P-labeled Bpa217 TFAM and WT (lanes 1 and 2) or Trp-122 mtRNAP (lanes 3 and 4). Lower panel, cross-linking performed as above but using 32P-labeled Bpa228 TFAM. d, MtRNAP interacts with the −49 base of the HSP promoter. TS, DNA template strand; NT, DNA non-template strand. e, transcription initiation complexes assemble in close proximity on human mtDNA. Interactions probed in this study are indicated by the yellow stars.