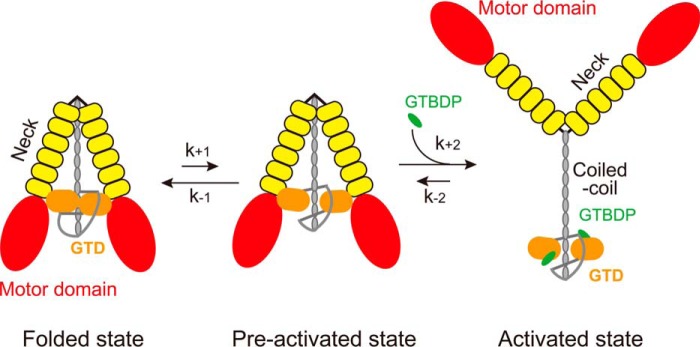
FIGURE 6.
Proposed mechanism for the activation of Myo5a by Mlph-GTBDP. The inhibited Myo5a molecules are equilibrated between the folded state and the preactivated state. In the folded state, the two GTDs form a head to head dimer via the interaction between the N-terminal extension in one GTD and the Mlph-GTBDP binding site in the counterpart GTD; the GTD dimer interact with the two heads of Myo5a, thus forming a folded, triangular conformation. In the preactivated state, Myo5a also forms a folded, triangular conformation as in the folded state, except that the Mlph-GTBDP binding sites in the GTDs are exposed. Mlph-GTBDP is able to bind to the GTD of Myo5a in preactivated state and allosterically inhibits the binding of GTD to the head, thus inducing the extended conformation and activating the motor function of Myo5a.