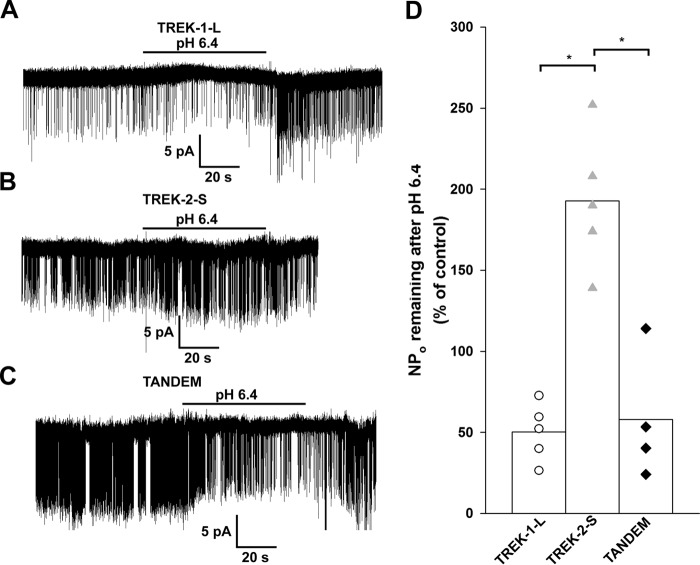
FIGURE 6.
Effect of extracellular acidification on single TREK channels. TREK-1-L, TREK-2-S, and TREK-2-S/TREK-1-L tandem channels were expressed in Xenopus oocytes. Single channel currents were recorded in symmetrical 140 mm K+ from outside-out membrane patches. Current traces displayed were filtered at 2 kHz. A–C, representative current recordings of outside-out membrane patches from Xenopus oocytes expressing TREK-1-L (A), TREK-2-S (B), and TREK-2-S/TREK-1-L tandem (C) at a membrane potential of −60 mV. Acidification of the bath (pH 7.4 to pH 6.4) is indicated by the horizontal bar. The effect of the decrease in the extracellular pH was reversible by switching back the pH of the bath medium to pH 7.4. Channel activity (NPo) was determined from 30 to 60 s of recordings before, during, and after acidification of the bath. D, effect of extracellular acidification on channel activity of TREK-1-L (white circles, n = 5 patches), TREK-2-S (gray triangles, n = 5 patches), and TREK-2-S/TREK-1-L (black diamonds, n = 4 patches) channels is displayed as a scatterplot. The averages of the groups are plotted as columns. The difference between the effect of extracellular acidification on the channel activity of TREK-2-S compared with TREK-1-L and TREK-2-S/TREK-1-L was found to be statistically significant (p < 10−3). The difference between the effect of extracellular acidification on the channel activity of TREK-1-L and TREK-2-S/TREK-1-L was not significant (p = 0.94). * represents significant differences.