FIGURE 8.
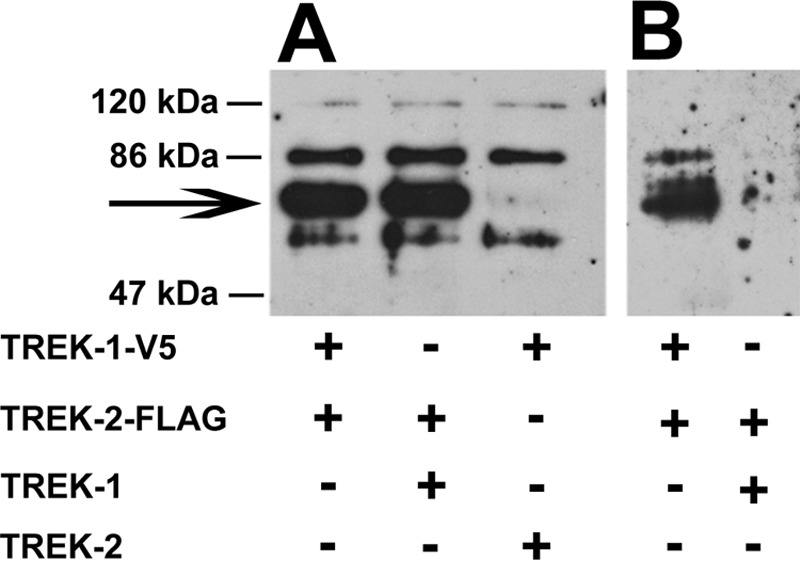
Coimmunoprecipitation of TREK-1 and TREK-2 subunits. Xenopus oocytes were coinjected with cRNA of TREK-1 and TREK-2 channel constructs, in different combinations of wild type or epitope-tagged forms, as indicated. A, total membrane lysates were prepared, solubilized, and subjected to SDS-PAGE. Size-fractionated proteins were transferred to nitrocellulose membrane; the blots were probed with monoclonal anti-FLAG antibody, and visualized by HRP-conjugated anti-mouse antibody. The arrow marks the specific TREK-2-FLAG immunoreactivity. B, coimmunoprecipitation was performed on solubilized membrane preparations using beads to which polyclonal anti-V5 antibody was bound. The affinity-purified proteins were analyzed as described at A. The blot shown is representative of three independent experiments.
