FIGURE 6.
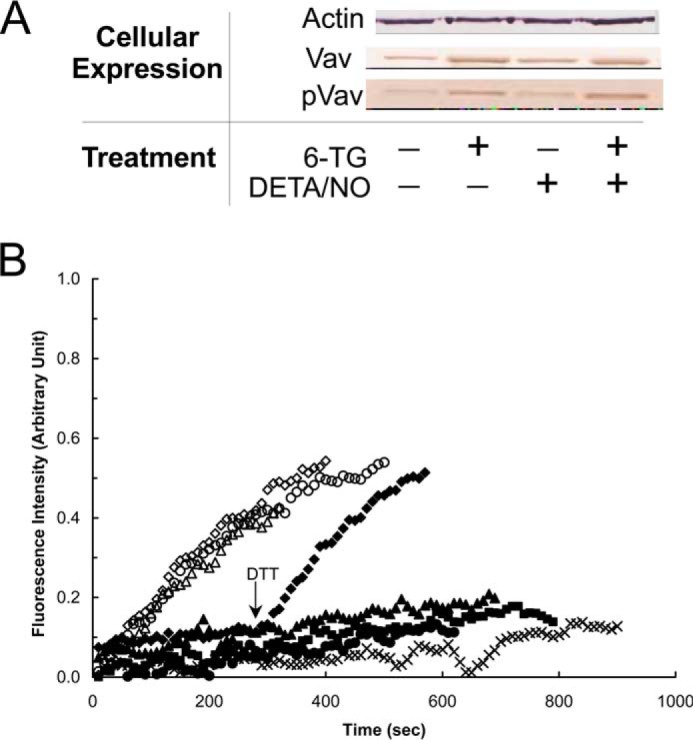
6-TG enhances the expression and phosphorylation of Vav but does not inhibit the specific activity of Vav in CD4+ cells. A, Western analyses were performed for Vav and pVav, respectively, by using anti-Vav and anti-pVav antibodies as described under “Experimental Procedures.” Actin expression was shown as a control. B, fluorescence mant-based kinetic properties of the 6-TGNP-Rac1 adduct, in comparison with the GDP-Rac1, in the presence and absence of various Vav proteins, were examined as described under “Experimental Procedures.” Both the GDP-bound Rac1 and the 6-TGDP-Rac1 adducts were used as substrates of Vav. Three different forms of Vav proteins also were used as follows: (i) Vav from nontreated; (ii) treated only with 6-TG; and (iii) 6-TG and DETA/NO treated CD4+ cells. Accordingly, six different kinetic assays were conducted as follows: the GDP-bound Rac1 with Vav from nontreated CD4+ cells (▵); the GDP-bound Rac1 with Vav from CD4+ cells treated with 6-TG (♢); the GDP-bound Rac1 with Vav from CD4+ cells treated with both 6-TG and DETA/NO (○); the 6-TGDP-Rac1 adduct with Vav from nontreated CD4+ cells (▴); the 6-TGDP-Rac1 adduct with Vav from CD4+ cells treated with 6-TG (♦); and the 6-TGDP-Rac1 adduct with Vav from CD4+ cells treated with both 6-TG and DETA/NO (●). The kinetic assay controls, the GDP-bound Rac1 without Vav (■) and the 6-TGDP-Rac1 adduct without Vav (×), were also conducted. Note that DTT (10 mm) was added to the assay cuvette containing the 6-TGDP-Rac1 adduct with Vav from CD4+ cells treated with 6-TG at time 300 s as indicated by an arrow. All fluorescence spectra were normalized against the spectrum of the GDP-bound Rac1 with Vav from 6-TG and DETA/NO treated CD4+ cells because it shows the highest fluorescence intensity at time 900 s.
