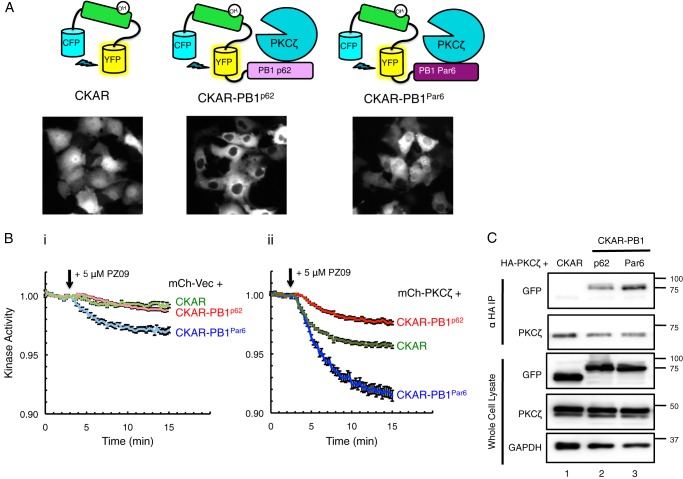
FIGURE 2.
PKCζ is constitutively active on CKAR substrate reporter tethered to interacting PB1 domains of scaffold proteins p62 and Par6. A, diagram of reporters constructed to measure aPKC activity: CKAR, CKAR-PB1p62, and CKAR-PB1Par6, with corresponding images of COS-7 cells transfected with each reporter showing its localization. B, basal kinase activity of mCherry-Vec (mCh-Vec) (panel i) and mCh-PKCζ (panel ii) on each reporter shown in A after treatment with 5 μm PZ09 in live COS-7 cells. The trace for each cell imaged was normalized to its t = 0-min baseline value and plotted as mean ± S.E. Normalized FRET ratios were combined from four independent experiments (CKAR-PB1Par6 traces) or five independent experiments (CKAR and CKAR-PB1p62 traces), with each experiment analyzing 4–12 selected cells. C, HA-PKCζ was co-expressed with CKAR, CKAR-PB1p62, or CKAR-PB1Par6 in COS-7 cells, immunoprecipitated (IP) from soluble lysates using anti-HA antibody, and blotted for co-IP of CKAR tag using anti-GFP antibody; whole cell lysate was loaded at 10% input.