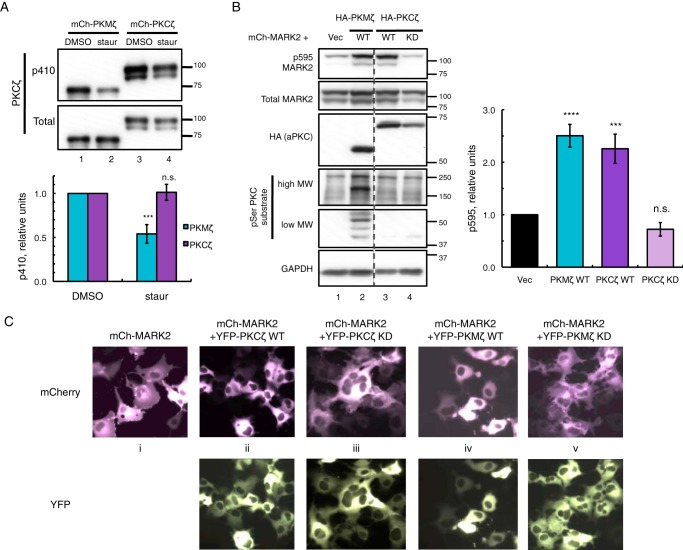
FIGURE 5.
PKMζ is more sensitive to dephosphorylation than PKCζ and active on global substrates, although both are basally active on MARK2 substrate. A, immunoblots showing activation loop phosphorylation (p410 PKCζ) of mCherry (mCh)-PKMζ versus mCherry-PKCζ expressed in COS-7 cells treated with either DMSO vehicle or 1 μm staurosporine (staur) for 2 h prior to lysis. The p410/total PKCζ ratios were quantified from four (PKMζ) or five (PKCζ) independent experiments, normalized to DMSO controls, and plotted as mean ± S.E. Statistical analysis was performed using two separate t tests comparing staurosporine treatment versus DMSO control for each protein. Significance was notated as ***, p < 0.001, or n.s., not significant. B, immunoblots of COS-7 co-expressing mCherry-MARK2 and either vector, HA-tagged wild-type (WT) PKMζ, or PKCζ or kinase-dead (KD) PKCζ. The p595/total MARK2 ratios were quantified from six (PKCζ KD) or seven (Vec, PKCζ WT, and PKMζ WT) independent experiments, normalized to Vec-transfected control and plotted as means ± S.E. Statistical analysis was performed using ordinary two-way analysis of variance followed by Dunnett's multiple comparison test with Vec as control. Significance notated as **** (p < 0.0001); *** (p < 0.001); or n.s., not significant. C, images of live COS-7 expressing mCherry-MARK2 alone or co-expressed with YFP-tagged WT or KD versions of PKMζ or PKCζ.