Abstract
Since their discovery, nanobodies have been used extensively in the fields of research, diagnostics and therapy. These antigen binding fragments, originating from Camelid heavy-chain antibodies, possess unusual hallmarks in terms of (small) size, stability, solubility and specificity, hence allowing cost-effective production and sometimes outperforming monoclonal antibodies. In this review, we evaluate the current status of nanobodies to study, diagnose, visualize or inhibit cancer-specific proteins and processes. Nanobodies are highly adaptable tools for cancer research as they enable specific modulation of targets, enzymatic and non-enzymatic proteins alike. Molecular imaging studies benefit from the rapid, homogeneous tumor accumulation of nanobodies and their fast blood clearance, permitting previously unattainable fast tumor visualization. Moreover, they are endowed with considerable therapeutic potential as inhibitors of receptor-ligand pairs and deliverers of drugs or drug-loaded nanoparticles towards tumors. More in vivo and clinical studies are however eagerly awaited to unleash their full potential.
Keywords: Single domain antibody, Heavy chain antibody, VHH, Nanobody, Cancer
Highlights
-
•
Nanobodies can be applied as highly specific diagnostic agents and molecular imaging probes.
-
•
Their intracellular functionality enables analysis and prediction of interesting therapeutic intervention points.
-
•
Therapeutic potential is high and can be obtained with bare, ionizing, or drug- and particle-guiding nanobodies.
1. Introduction
Over the last decades, monoclonal antibodies (mAbs) against cancer-related transmembrane receptors, or their ligands, have found their way to the clinic. mAbs can direct a cytotoxic payload towards tumor cells, or radioactive or fluorescent tracers for PET/SPECT or optical imaging, respectively. Their distribution and tumor penetration are however limited due to mAb dimensions (~ 150 kDa, 10–15 nm long and 7–9 nm wide). Moreover, their long half-life (ranging from days to up to 4 weeks) accounts for high background levels during molecular imaging. In addition, host immune responses still remain an issue.
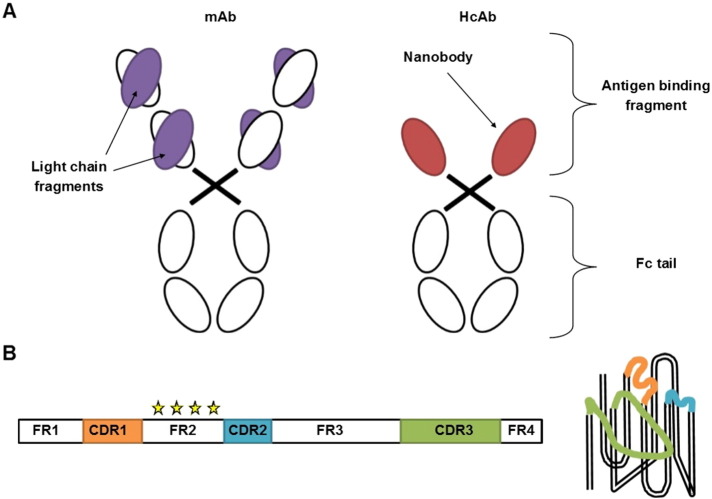
The variable fragments of Camelid heavy-chain only antibodies (HcAbs), called nanobodies, may provide an answer to several of these concerns (Hamers-Casterman et al., 1993) (Fig. 1A). Nanobody hallmarks include small size (~ 15 kDa, 4 nm long and 2.5 nm wide), high solubility, stability, specificity and affinity, ease of cloning as well as thermal and chemical resistance. Moreover, recombinant production in microorganisms is very cost-efficient and nanobodies can easily be used as building blocks for multi-domain constructs (Muyldermans, 2013). These advantageous properties arise from their single domain nature and from crucial amino acid mutations in the framework 2 region, rendering the overall structure more hydrophilic compared to conventional antibody fragments (Fig. 1B). Their convex surface and extended CDR3 loop further enables recognition of cavities or hidden epitopes on the surface of the antigen (Fig. 1B). Combined with the fact that nanobodies are considered to be non-immunogenic due to their high similarity with human VH sequences, these unique properties triggered numerous applications in fundamental research, diagnostics and therapy (De Meyer et al., 2014, Chakravarty et al., 2014, Kijanka et al., 2015, Muyldermans, 2013, Oliveira et al., 2013).
Fig. 1.
Representation of a heavy-chain antibody (HcAb) and its antigen binding fragment, called nanobody. A. In contrast to a monoclonal antibody (mAb), which comprises two heavy and two light chains, an HcAb only contains heavy chains. As HcAbs also lack one constant domain, the antigen binding region only consists of a single fragment, called a nanobody. The tail region of the antibodies forms the Fc part and is able to trigger the immune system. B. Schematic representation (left) and conformation (right) of the nanobody entity, composed of framework regions (FR1–4) alternated with three complementary determining regions (CDR1–3). Mutations in FR2 (stars) render the structure more hydrophilic as compared to conventional antibody fragments. Moreover, the CDR3 loop is extended and enables recognition of hidden or buried epitopes.
Nanobodies are stable in the reducing cytoplasmic environment and when expressed as an intrabody they can modulate, trace and visualize antigens (Muyldermans, 2013, De Meyer et al., 2014). Moreover, they can serve as biomarker probes and when fused to radionuclides or near-infrared fluorophores they represent ideal non invasive in vivo imaging agents (Chakravarty et al., 2014, Oliveira et al., 2013). Therapeutically, they can be utilized as neutralizing agents, as receptor-ligand antagonists and as vehicles for effector delivery or targeted vehicle-based drug therapy (Kijanka et al., 2015, Oliveira et al., 2013). Their development as antagonists of extracellular disease-related targets is currently undergoing phase I, II and III clinical trials by Ablynx, the company of which nanobodies are the trademark (http://www.ablynx.com). Although nanobodies also aid in identifying new interesting intracellular targets, their penetration through the cell membrane remains a problematic issue for therapeutic targeting of cytosolic proteins. In this review, we provide insight into the current status, ongoing developments and future challenges towards successful implementation of nanobodies in the diagnosis and treatment of cancer.
2. Therapeutic Nanobodies Directed Against Extracellular Targets
In addition to ‘classical’ receptor targets such as EGFR (Roovers et al., 2007,Roovers et al., 2011, Schmitz et al., 2013, Omidfar et al., 2013), HER2 (Jamnani et al., 2012, Even-Desrumeaux et al., 2012), c-MET (Slordahl et al., 2013) and VEGFR (Behdani et al., 2012), nanobodies against new targets such as the DR5 death receptor (Huet et al., 2014, Papadopoulos et al., 2015) and the chemokine receptors CXCR4 (Jahnichen et al., 2010) and CXCR7 (Maussang et al., 2013, Blanchetot et al., 2013) come into play. Alternatively, nanobodies can be generated against the cognate receptor ligands, such as HGF (for c-MET) (Vosjan et al., 2012), VEGF (for VEGFR) (Kazemi-Lomedasht et al., 2015, Ebrahimizadeh et al., 2015, Farajpour et al., 2014), uPA (for uPAR) (Kaczmarek and Skottrup, 2015) or CXCL11/12 (for CXCR7) (Blanchetot et al., 2013) (Table 1).
Table 1.
Overview on the distinct nanobody-based applications, their advantages and drawbacks when applied as therapeutics, drug delivery moieties, intrabodies, diagnostics and/or imaging tools. The different constructs discussed in this review are summarized as well as the reported issues and proposed solutions for each particular application. See main text for more details and accompanying references. At right, the current status in terms of conducted in vitro, in vivo and clinical experiments is given.
| Nanobody constructs | Nanobody advantages | Reported issues | Solution | Current status | |
|---|---|---|---|---|---|
| Nanobodies against extracellular targets |
Receptors as target EGFR, HER2, c-MET, VEGFR, DR5, CXCR4/7 Ligands as target HGF, VEGF, uPA, CXCL11/12 |
Excellent domain building blocks Deep & homogenous tumor accumulation Target new epitopes |
Low affinity Fast blood clearance Lack of Fc Immunogenicity |
Nb mixtures or multivalent constructs Fusion to anti-Albumin nanobody Adding an Fc-tail Humanization |
In vitro +++ In vivo xenografts ++ Clinical trials + (aborted) Therapeutic potential |
| Nanobodies for drug delivery |
Targets VEGFR2, EGFR, c-MET, HER2, MUC1 Toxins to deliver Pseudomonas exotoxin A Particles to deliver Liposomes, micelles, NANAPs, polymersomes, polyplexes |
Suited for conjugation No Fc tail Rapid tumor accumulation Can act antagonistic itself |
Poor solubility and/or stability of drugs Fast blood clearance Affecting mostly cell growth/proliferation |
Encapsulation in nanoparticles PEGylation Aiming for cell death effect |
In vitro +++ In vivo xenografts ++ Clinical trials / Therapeutic potential |
| Study of intracellular protein function |
Intracellular targets Fascin, cortactin, CapG β catenin, PKCε Vimentin, GFP |
Intracellular stability/activity Domain/function specificmodulation Easy to fuse: use as chromo or deloc constructs Aid in drug discovery/rational drug design |
Cell membrane penetration required for therapeutic use |
Use of EPEC Spontaneously penetrating nanobodies |
In vitro +++ In vivo xenografts + Applicable for cell biological research |
| Nanobodies to detect cancer biomarkers |
Extracellular biomarker targets AFP, CAIX, PMSA TAG-72, HER2 |
High stability Easy to conjugate Suitable in several applications: ELISA, PCR, IHC Small size, enabling chip format Reveal new intracellular biomarkers for IHC |
Increased performance desired |
Application specific Use nanobody mixture |
In vitro +++ Applicable for diagnostic purposes |
| Nanobodies for molecular imaging |
Targets PMSA, MMR, HER2, HGF, VCAM1, CAIX, EGFR Labeling 99mTc, 177Lu, 111In, 123I, 68Ga, 89Zr, 124I, 131I Micro/nanobubbles IRDye800CW, IRDye700DX |
Rapid & homogenous tumor accumulation Fast blood clearance Easy conjugation Early imaging, safe procedure Combination imaging & therapy possible Image-guided surgery becomes possible |
Accumulation in kidneys Accumulation in liver and spleen Accumulation in liver and intestines |
Remove His-tag Co-inject gelofusin and/or lysine Change chelating agent Construct-specific Administerunlabeled Nb Later time point of imaging |
In vitro +++ In vivo xenografts +++ In vivo mouse models +++ Clinical trials + (successful) Applicable for imaging Therapeutic potential |
Generally, one starts from a pool of nanobodies against the desired target. Further selection is based on nanobody affinity (nM) and the capacity to inhibit receptor-ligand binding or receptor activity in vitro. Higher affinity or avidity may be obtained by using a mixture of nanobodies recognizing different epitopes at the surface of the same antigen (oligoclonal) (Jamnani et al., 2012) or by using multivalent nanobodies (Even-Desrumeaux et al., 2012, Huet et al., 2014), which are usually linked in tandem via flexible glycine-serine linkers (Maussang et al., 2013, Huet et al., 2014). In addition, the nanobodies are often evaluated against characterized mAbs by competition assays. Remarkably, an anti-EGFR nanobody did not compete with Cetuximab but structural studies demonstrated that it targets an epitope that would not be accessible for the flatter mAb paratope, pointing to the advantage of nanobodies to reveal new intervention points (Schmitz et al., 2013).
To predict nanobody therapeutic efficacy, preclinical cancer cell line models are utilized in diverse experimental settings such as cell adhesion, proliferation, migration, angiogenesis-like properties or perturbation of specific signaling pathways. The small size of nanobodies is conducive to deep(er) and homogenous tumor penetration but disadvantageous in terms of in vivo half-life (few hours). Therefore, nanobodies are often linked to an anti-albumin nanobody, enabling binding to serum albumin (~ 66 kDa) (Tijink et al., 2008, Vosjan et al., 2012, Slordahl et al., 2013, Roovers et al., 2011, Maussang et al., 2013). Several successful nanobody-based in vivo xenograft studies with bispecific or multivalent nanobodies were reported, resulting in delay of tumor growth (Vosjan et al., 2012, Roovers et al., 2011) or inhibition of angiogenesis (Maussang et al., 2013). Such constructs sometimes outperform the corresponding mAb (Huet et al., 2014) but in other cases they don't. For instance, a CONAN-1 nanobody could not outperform Cetuximab, probably due to the lack of an Fc region and associated immune effector functions (Roovers et al., 2011). Adding an Fc tail, as done before for other nanobodies (De Buck et al., 2013), could provide a solution, but is not yet generally established. Although nanobodies are considered non-immunogenic, the generation of antibodies against administrated nanobodies can be an issue, as shown in an (aborted) clinical trial with anti-DR5 receptor nanobody (Papadopoulos et al., 2015). Moderate humanization can be performed to anticipate potential immune responses (Slordahl et al., 2013). Despite the high potential of nanobodies targeting other ligand-receptor combinations such as VEGF-VEGFR, in vivo studies for those targets are still wanting.
In summary, while nanobodies are advantageous in terms of economical production, possibility of targeting unique epitopes and multimerization capacity, the lack of an Fc tail or a lower affinity can sometimes render them less potent than their mAb counterparts. Humanization might be required in some cases. However, as in vivo studies and clinical trials are still lacking for most cases, comparison with established mAbs is not yet fully possible (Table 1).
3. Nanobody-mediated Drug Delivery
Several researchers exploited currently available nanobodies against tumor-specific receptors as conduits for delivering toxins or drugs to tumors, thereby reducing nonspecific toxicity to normal cells and diminishing side effects. The lack of an Fc tail is advantageous here, as Fc-mediated clearance or a triggered immunogenic response might prevent delivery of the cargo. A recent example of an immunotoxin is the dimeric construct composed of an anti-VEGFR2 nanobody and a truncated form of the Pseudomonas exotoxin A (PE38), cloned with a linker in between (Behdani et al., 2013) (Table 1).
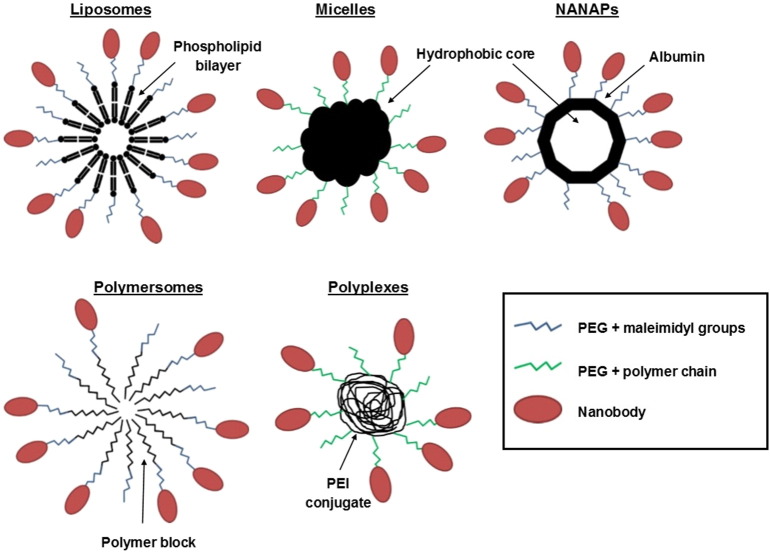
Most efforts are done in the field of nanoparticles (diameter < 200 nm), as encapsulation of drugs further overcomes problems such as poor solubility, limited stability and rapid clearance (Table 1). Carriers used include liposomes (Oliveira et al., 2010, van der Meel et al., 2012,2013), micelles (Talelli et al., 2011,2013), albumin-based nanoparticles (NANAPs) (Altintas et al., 2013, Heukers et al., 2014a) and polymer-based polymersomes (Zou et al., 2015) or polyplexes (Sadeqzadeh et al., 2011) (Fig. 2). PEGylation is generally applied to extend blood half-life and the nanobody can be conjugated at a maleimidyl group or at introduced polymer chains at the distal end of the hydrophilic PEG block. The nanobody is chemically modified with N-succinimidyl-S-acetylthioacetate (SATA) or coupled via cysteine chemistry. Both methods do generally not affect nanobody binding capacity (Heukers et al., 2014a, Massa et al., 2014). On-site accumulation of nanoparticles is facilitated by enhanced permeability and retention (EPR), as tumors bear leaky blood vessels and defective lymph drainage. Moreover, due to the presence of several nanobodies on the same particle surface, avidity comes into play (Huet et al., 2014). Mostly applied for several particle formats is the anti-EGFR nanobody EGa1, which acts antagonistically towards EGFR and thus not only contributes to drug guidance but also to the therapeutic effect.
Fig. 2.
Overview on nanoparticle types that can be decorated with nanobodies to mediate selective targeting. Liposomes are based on lipid bilayers, while micelles and albumin-based NANAPs provide a hydrophobic core to encapsulate drugs. Polymersomes and polyplexes are synthetic polymer-based particles and represent relatively new vesicle types, of which the latter can be used to compact DNA plasmids. PEGylation is generally performed to extend blood half-life. Maleimidyl groups or polymer chains at the distal end of PEG enable conjugation with the nanobody.
Van der Meel and co-workers conjugated the anti-EGFR nanobody on the surface of liposomes, and this coincided with receptor-mediated internalization of EGFR and thus EGFR downregulation (van der Meel et al., 2012). Loading the liposomes with an anti-IGF-1R kinase inhibitor AG538 additionally affected IGF-1R signaling and further increased the inhibitory effect on cancer cell growth in vitro. However, significant growth inhibition was only observed for one of the two cell lines examined in a mouse xenograft model (van der Meel et al., 2013). The same nanobody was applied by Talleli et al. to coat the surface of thermosensitive, biodegradable polymeric micelles (Talelli et al., 2011). These micelles already intrinsically inhibit tumor growth in vivo, and this can be further increased by covalently encapsulating the chemotherapeutic doxorubicin (Talelli et al., 2013). Correspondingly, prolonged animal survival was observed. On the other hand, nanobody-albumin nanoparticles (NANAPs) are considered biocompatible and safe due to the abundance of the protein in serum. The multikinase inhibitor 17,864 was encapsulated in Ega1-coated NANAPs, resulting in clathrin-mediated endocytosis, digestion of the particles in the lysosomes, release of the inhibitor in the cytosol and reduced cancer cell proliferation in vitro (Altintas et al., 2013). Recently, also NANAPs decorated with anti-Met nanobody were shown to be transported to lysosomes for degradation, hence providing another system for lysosomal drug delivery (Heukers et al., 2014a).
Nanobody-mediated targeting of artificial polymer-based vesicles has also received increased attention in the last years. Both polymersomes based on biocompatible PEG-b-PCL and functionalized with anti-HER2 nanobody (Zou et al., 2015) as well as polyplexes composed of PEGylated PEI conjugates and decorated with anti-MUC1 nanobody (Sadeqzadeh et al., 2011) enable selective cancer cell targeting. Of note, the latter macromolecular entity enables compaction of DNA plasmid and hence ‘transcriptional’ drug delivery as shown for the truncated-Bid (tBid) killer DNA. When under control of a MUC1 promotor, administration of these DNA-containing polyplexes resulted in elevated Bid/tBid expression as well as considerable cell death (Sadeqzadeh et al., 2011).
Hence, nanobodies are very convenient tools for delivering toxic cargos to cancer cells and are well-suited for chemical conjugation onto different nanoparticle formats. Moreover, they can boost the therapeutic effect when bearing intrinsic antagonistic activity. However, in vitro and in vivo data are not yet available for all cases reported here and most of the effects concern inhibition of cell growth or proliferation rather than effective cell death. When completely established, clinical studies will have to shed light on the safety and efficacy of these nanobody-targeted carrier molecules (Table 1).
4. Nanobodies Against Intracellular Targets: Tools to Identify New Targets and Springboard to Future Therapeutics
As nanobodies do not suffer from the reducing cytoplasmic environment, they can be utilized as intrabodies. Unlike siRNAs, nanobodies provide the advantage of protein domain- and protein function-specific modulation (similar to the way drugs act), enabling high resolution protein analysis compared to complete protein downregulation or eradication (Table 1). For example, a nanobody that inhibits fascin-mediated actin bundling or a nanobody that targets the SH3 domain of the actin branch regulator cortactin demonstrated that fascin bundling activity is required for cancer cell invadopodium longevity whereas cortactin SH3 domain functionality underpins invadopodia formation and activity (Van Audenhove et al., 2014). Similarly, a nanobody that prevents CapG from regulating the length of actin filaments through capping the (+) ends of actin polymers was found to impair in vitro invasion. Moreover, this nanobody reduced lung metastasis of human breast cancer cell xenografts by more than 90% (Van Impe et al., 2013). Furthermore, nanobodies inhibiting β-catenin-mediated Wnt signaling (Newnham et al., 2015) and nanobodies inhibiting or activating PKCε kinase (Summanen et al., 2012) were shown to function intracellularly. They can be used in the future to gain insight into the respective pathways that they regulate. These types of nanobodies will contribute to a better understanding of protein structure-function relationships and might aid in the discovery or rational design of new therapeutic drugs through medicinal chemistry.
Deliberate intracellular protein displacement for molecular studies is relatively easy to achieve when considering a protein that is overexpressed, but less obvious to elicit for a resident endogenous protein. Tagging a nanobody with a targeting sequence allows its displacement, and that of its endogenous antigen, to virtually any compartment or region in the cell, enabling researchers to study the correlation between protein location and function. This strategy is also a good alternative when there are no nanobodies available that inhibit a particular function of the antigen. For example, a MOM (mitochondrial outer membrane) tag anchors the nanobody and its interaction partner at the mitochondria (van Audenhove et al., 2014, Van Impe et al., 2013). In this way, the endogenous protein can be ‘depleted’ from its normal site(s) of action. Moreover, intrabodies can be fused to fluorescent tags such as GFP, yielding chromobodies, to enable visualization of protein localization and behavior as shown for actin binding proteins as well as vimentin (van Audenhove et al., 2014, Maier et al., 2015). Finally, in the so-called fluorescent-three-hybrid strategy, a GFP targeting nanobody is anchored at a subcellular compartment such as the nucleus, while the proteins of interest (bait and prey) are fused with GFP and RFP, respectively (Herce et al., 2013). Interaction between bait and prey results in their colocalization at the site of the GFP-recognizing nanobody and this can be followed in real-time in living cells. As such, this model can be used to study protein-protein interactions or to select and evaluate inhibitors.
The observation that a nanobody targeting CapG, a cytoskeletal protein that is relatively unknown, drastically curtails breast cancer metastasis in an orthotopic in vivo xenograft model (Van Impe et al., 2013) is revealing. It suggests that there are many drug targets other than GPCRs or kinases/phosphatases, inhibition of which results in a more than sizable anti-metastatic effect. Moreover, targeting such downstream effectors may be less prone to eliciting side effects and we predict that there are many cytoplasmic proteins that are equally interesting from a therapeutic point of view as CapG. A major obstacle however is nanobody intracellular delivery (Table 1). Some recent advances provide the first clues to solve this problem. First, the type III protein-secretion system (T3SS) of enteropathogenic E.coli (EPEC) enables injection of nanobodies into the cytoplasm of (cancer) cells, as shown for the CapG-inhibiting nanobody (Van Impe et al., 2013). The genes encoding the filamentous injectisome have been transferred into the non-pathogenic E. coli K-12 strain and were shown to reconstitute into a functional structure (Ruano-Gallego et al., 2015). Second, Li and co-workers showed that nanobodies against GFAP (glial fibrillary acidic protein, an intermediate filament protein), a marker of astrocytes, can cross the blood-brain-barrier both in vitro and upon injection in vivo, resulting in diffusion into brain tissue and penetration into astrocytes (Li et al., 2012). This suggests that nanobodies can be spontaneously cell penetrating under some circumstances (high pI) but the observation as such necessitates independent confirmation by others.
Thus, to turn nanobodies against intracellular targets into potent therapeutics, future attempts should focus on their efficient and safe cellular membrane penetration. To obtain site-specific targeting, an interesting possibility that remains to be investigated is the encapsulation of recombinant nanobodies, or the compaction of nanobody plasmids, into nanoparticles, decorated with for example anti-EGFR nanobodies.
5. Detecting or Defining Cancer Biomarkers by Means of Nanobodies
Apart from therapy, nanobodies can aid in early diagnosis and cancer prevention by detecting or defining biomarkers (Table 1). Nanobodies can improve current mAb-based diagnostic techniques due to their high specificity. Furthermore, their high stability under extremes of temperature, pH, or ionic strength, as shown for the cancer biomarker alpha-fetoprotein (AFP) (Chen et al., 2016), ensures that the application still can occur under harsh conditions. Cell-based ELISA was successful for nanobodies against the carbonic anhydrase IX enzyme (CAIX) (Araste et al., 2014), prostate-specific membrane antigen PMSA (Zare et al., 2014), tumor-associated glycoprotein 72 (TAG-72) (Sharifzadeh et al., 2013) and HER2 (Jamnani et al., 2012). Of note, a better performance was achieved with a mixture of several nanobodies. To perform sandwich ELISA, both a capturing and detecting nanobody are used, targeting different epitopes on the antigen. Nanobodies against AFP reached a detection limit of 0.47 ng/mL. One step further, a chip format sandwich ELISA with anti-HER2 nanobodies covalently bound onto a screen-printed electrode (SPE) was proposed with a detection limit of 1 μg/mL (Patris et al., 2014). The most sensitive detection (0.0005 ng/mL) could be achieved by means of immune-PCR with anti-AFP nanobodies (Chen et al., 2016).
Due to the relative ease of single domain nanobody generation compared to conventional antibodies, an elegant and fast strategy to generate nanobodies against (unknown) cancer biomarkers is by performing immunization with patient samples. This strategy resulted in the identification of a new breast cancer-specific biomarker, cytokeratin 19 (Even-Desrumeaux et al., 2012). Similarly, the technology of nanobody-based reverse proteomics was used in glioblastoma multiforme (GBM) (Jovcevska et al., 2014). By performing mass-spectrometry analysis on nanobody-antigen pairs, the new GBM biomarkers TRIM28 and β-actin could be revealed. As all these markers are localized intracellularly, they are especially proposed for immunohistochemical-based diagnostic purposes.
6. Nanobodies in Molecular Imaging
The small size of nanobodies is highly advantageous especially in the field of molecular imaging as it enables rapid tumor accumulation and homogenous distribution as well as efficient blood clearance, contributing to high tumor-to-background ratios. Moreover, nanobodies can be easily conjugated to several kinds of imaging agents and their high specificity renders their use relatively safe.
Single-photon emission computed tomography (SPECT) is based on γ-rays and nanobodies are here linked to radionuclides such as 99mTc, 177Lu, 123I and 111In. On the other hand, the positron-emitting radioisotopes 68Ga, 124I or 89Zr are used for positron emission tomography (PET) purposes. Again, especially cancer-specific receptors such as EGFR (Vosjan et al., 2011) and HER2 (D'Huyvetter et al., 2012, D'Huyvetter et al., 2014, Xavier et al., 2013, Keyaerts et al., 2015, Massa et al., 2014, Pruszynski et al., 2013, Pruszynski et al., 2014) (Table 1) are interesting targets for tumor visualization. Recent research in this area further focuses on PMSA (Chatalic et al., 2015, Evazalipour et al., 2014), which is overexpressed in prostate cancer, and HGF (Vosjan et al., 2012), which is implicated in several cancers, though also in cardiovascular disease. Alternatively, Movahedi and coworkers raised nanobodies against the macrophage mannose receptor MMR, which is highly expressed by tumor-associated macrophages and thus serves as an alternative strategy to image the tumor stroma, especially the hypoxic areas (Movahedi et al., 2012).
Prior to radiolabeling, nanobodies are conjugated with bifunctional chelating agents which possess a metal binding moiety for sequestration of the metallic radionuclide and are generally DPTA (acyclic)-, DOTA (macrocyclic), or NOTA-based. Moreover, the chelating agents are equipped with a chemically reactive functional group for attachment to the nanobody, which can occur in several ways. First of all, random conjugation can be performed via the free ε-amino-group on nanobody lysines, as shown for the anti-HER2 and the anti-PMSA nanobodies. Second, Massa and co-workers demonstrated a generic strategy for site-specific labeling of nanobodies (Massa et al., 2014). To this end, the anti-HER2 nanobody was cloned with a C-terminal unpaired cysteine following its hexahistidine tag. A reduction step was needed prior to conjugation due to spontaneous dimerization and capping of the unpaired cysteine. Subsequently, the reduced probe could be bound to maleimide-DPTA, followed by labeling with 111In. Third, the hexahistidine tag of the nanobody can be directly labeled with 99mTc(CO)3 without any chemical modification of the protein, as shown for an anti-PMSA nanobody and the anti-MMR nanobody (Evazalipour et al., 2014, Movahedi et al., 2012). Site-specific or His-mediated labeling has the advantage that antigen binding activity of the nanobody is usually unaffected. When lysine residues involved in random conjugation are located outside the antigen-binding loops, no interference with antigen binding should be expected either (Xavier et al., 2013, Keyaerts et al., 2015).
Selection of ‘lead’ components for further (pre)clinical testing usually occurs on the basis of production yield, affinity and specificity, which should always be re-evaluated in their (radionuclide) labeled format. When internalization in cell line models is established, final validation is done in vivo with respect to tumor uptake, tumor-to-normal organ ratios and fast blood clearance. As such, selected components enable imaging as early as 1 h post-injection, which contributes to patient safety.
One drawback of this approach is the accumulation of radiolabeled nanobodies in the kidneys and bladder (Table 1), which is in fact a typical characteristic for small radiolabeled proteins and peptides. After glomerular filtration (cut-off of 60 kDa), nonspecific reabsorption in the proximal tubuli may account for the residence in kidneys. Remarkably, D'Huyvetter and co-workers showed that the highest accumulation occurs with Myc-His-tagged anti-HER2 nanobody, followed by His-tagged and finally untagged nanobody (70–88% less accumulation). The amino acid composition and polarity at the C-terminus thus predominantly affect kidney retention (D'Huyvetter et al., 2014). This could be diminished with 90–95% by co-injecting gelofusine, an inhibitor of the megalin receptor responsible for tubular protein reabsorption. Similarly, Chatalic and co-workers showed that renal uptake of their His-tagged anti-PMSA nanobody could be reduced without loss of targeting by co-injecting a combination of gelofusine and the positively charged amino acid lysine or by removing the His-tag from the nanobody (Chatalic et al., 2015). Also the untagged 68Ga-labeled anti-HER2 nanobody showed a 50% drop in renal accumulation compared to its His-tagged equivalent (Xavier et al., 2013). Another determining parameter can be the type of chelating agent, as significantly higher kidney uptake was reported for the DOTA-based conjugates compared to the DTPA-based conjugates of anti-HER2 nanobody (D'Huyvetter et al., 2012). Finally, albumin binding via a second nanobody could be a strategy to reduce renal retention, although this option is not preferred due to increased blood residence time and thus increased radiation exposure (Vosjan et al., 2012). For the case of 99mTc-labeled anti-MMR nanobodies to visualize tumor-associated macrophages, the accumulation was higher in liver and spleen (expressing MMR) as compared to the tumor (Movahedi et al., 2012). The authors solved this by administering an excess of unlabeled bivalent anti-MMR nanobody, which eliminates extratumoral signals while maintaining the targeting of tumor-associated MMR-positive cells.
Currently, the best established nanobody-based imaging agent is the 68Ga-coupled anti-HER2 nanobody 2Rs15d for PET imaging (Xavier et al., 2013). Preclinical evaluation revealed a good toxicological profile and a low radiation burden, enabling the construct to enter phase I clinical trials on humans (Keyaerts et al., 2015). The construct scored well in terms of efficiency, tracer accumulation and safety as no adverse effects or antibodies against the administrated nanobody were detected, rendering this construct suitable to enter phase II clinical trials. One drawback however was the uptake of the agent in liver and intestines, next to its expected accumulation in the kidneys. This might obscure liver metastasis, although no such metastases were present in the patient group and the question could thus not be solved. As the liver uptake decreases over time, a later time point of imaging is proposed by the authors to improve signal-to-noise ratio at the liver if needed (Keyaerts et al., 2015).
When using β-emitting radioisotopes such as 131I and 177Lu, a therapeutic effect can also be pursued by their ionization and DNA damaging activity. Such a radionuclide-based construct enabling both imaging and therapeutic use is termed ‘theranostic’. Gelofusin coinfusion with untagged 177Lu-DTPA-anti-HER2 nanobody almost completely blocked tumor growth in xenograft mouse models bearing small tumors, coinciding with increased event-free survival (D'Huyvetter et al., 2014). Moreover, non-specific radiation to healthy tissues was absent. Although the system causes a comparable radiation level for both tumor and kidneys, histopathological analyses of renal tissue revealed no visible toxicity, although long-term events cannot be excluded (D'Huyvetter et al., 2014). Characterization of this agent should be continued in clinical trials to highlight the potential of radiolabeled nanobodies as a valuable therapy. Also the radio-iodinated 131I-anti-HER2 nanobody shows potential for treatment of HER2-overexpressing malignancies (Pruszynski et al., 2013). Residualizing agents are used for radioiodination to avoid lysosomal degradation after receptor-mediated internalization, which would result in rapid loss of radioactivity. Such agents (for example containing guanidine or d-glutamates) render the construct ‘charged’ in lysosomes to prevent its diffusion across membranes and further avoid action of deiodinases. However, also very high radioactive levels were observed in the kidneys for the resulting constructs. The authors showed later on that radio-iodination via another method was superior in terms of tumor retention and coincided with lower uptake in normal tissues including the kidneys (Pruszynski et al., 2014), pointing to the importance of optimizing the labeling method.
Tumor imaging can also be performed by means of ultrasound waves, which are reflected differently according to the organ or tissue. Microbubbles are generally used as contrasting agents and nanobody-mediated guidance can further aid in improving contrast at the desired location. Recent examples include microbubbles decorated with anti-VCAM-1 nanobody for detection in the tumor vasculature (Hernot et al., 2012), and nanobubbles with anti-PMSA nanobody targeting PMSA-positive cells in vitro as well as in a mouse xenograft (Fan et al., 2015).
To avoid ionizing radiation, nanobodies can also be conjugated to near-infrared fluorophores such as IRDye800CW to perform optical tumor imaging, a technique that is cost-effective, flexible, sensitive and fast. As such, a nanobody against the hypoxia marker enzyme CAIX and an anti-EGFR nanobody were used for molecular imaging of (pre-) invasive breast cancer and orthotopic oral squamous cell carcinoma (OSCC), respectively (van Brussel et al., 2015, van Driel et al., 2014) (Table 1). The brightest future of this technique lies in imaging-guided surgery, which was first shown with anti-HER2 nanobodies. Under the guidance of real-time fluorescent images, surgical resection of a HER2-positive mouse xenograft could be demonstrated (Kijanka et al., 2013). Also for CAIX and EGFR targeting, tumor margins could be delineated already 2 h after nanobody administration with good tumor-to-background ratios in mouse models (van Brussel et al., 2015, van Driel et al., 2014). When using a photosensitizer fluorophore instead, such as IRDye700DX, specific cell death can even be induced upon light-mediated activation (Heukers et al., 2014b).
From the panoply of nanobody-based technologies directed at diagnosis and/or cancer treatment, the use of nanobodies in molecular imaging is the most promising due to its combination of all the advantages compared to current mAb based techniques, the swiftness and the safety of the procedure and the current status of this application (Table 1). Moreover, high nanobody-mediated specific radioactive deposition renders these constructs extremely suitable for therapeutic action on micrometastases and minimal residual disease.
7. Outstanding Questions
Although the potential of nanobodies against extracellular targets is high, both in the form of antagonistic nanobodies or nanobody-decorated nanoparticles (Table 1), further (pre)clinical studies should be performed to reveal their true potential in the clinic. The first clinical trials with anti-receptor or anti-ligand nanobodies apart from the DR5 receptor are eagerly awaited.
Especially the combination with transcriptional targeting via complexed plasmid deserves more attention, as this would open up a new era of applications. Moreover, this could aid in the still unsolved cell penetration problem for nanobodies against intracellular targets. Plasmid delivery via a safe drug carrier such as albumin-based NANAPs would circumvent many issues and enable delivery of virtually all kinds of constructs.
The usefulness of nanobodies targeting cytoplasmic proteins in general should not be underestimated. Researchers have found their way to these nanobodies for use in super-resolution microscopy in their recombinant format (Mikhaylova et al., 2015). As such, they allow single-particle trafficking to study protein function, localization and dynamics with a previously unattainable precision. Furthermore, as intrabodies, they show advantages compared to siRNA in cell biological research due to the ability to target domain-specific functions. Although this is not directly applicable in the clinic, these studies can indirectly aid in rational drug design by revealing the residues or surfaces that should be targeted by a small compound to mimic the effect triggered by the nanobody, hence accelerating drug development. More generally, X-ray co-crystal structures between a nanobody and its antigen, be it a cytoplasmic protein or transmembrane receptor, is thought to help drug development via medicinal chemistry but the first such compound remains to be discovered. Ultimately, such considerations would not be required if the membrane would be permeable to a nanobody. Despite isolated cases, this is not a general feature and chemical modifications will possibly be inevitable.
8. Search Strategy and Selection Criteria
Data for this Review were identified by searches of Sciencedirect, NCBI, PubMed, and references from relevant articles using the search terms “cancer” or “tumor” and “nanobody” or “VHH” in the abstract, title or keywords. Only articles published in English between 2010 and 2016 were included.
Acknowledgements
This work was supported by grants from the Research Foundation Flanders (Fonds Wetenschappelijk Onderzoek (FWO) Vlaanderen), Ghent University (BOF-GOA), and the Interuniversity Attraction Poles Programme of the Belgian State, Federal office for Scientific, Technical and Cultural Affairs (IUAP P7/13). IVA is supported by the FWO-Flanders.
References
- Altintas I., Heukers R., van der Meel R., Lacombe M., Amidi M., Van Bergen En Henegouwen P.M., Hennink W.E., Schiffelers R.M., Kok R.J. Nanobody-albumin nanoparticles (NANAPs) for the delivery of a multikinase inhibitor 17864 to EGFR overexpressing tumor cells. J. Control. Release. 2013;165:110–118. doi: 10.1016/j.jconrel.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Araste F., Ebrahimizadeh W., Rasooli I., Rajabibazl M., Mousavi Gargari S.L. A novel VHH nanobody against the active site (the CA domain) of tumor-associated, carbonic anhydrase isoform IX and its usefulness for cancer diagnosis. Biotechnol. Lett. 2014;36:21–28. doi: 10.1007/s10529-013-1340-1. [DOI] [PubMed] [Google Scholar]
- Behdani M., Zeinali S., Khanahmad H., Karimipour M., Asadzadeh N., Azadmanesh K., Khabiri A., Schoonooghe S., Habibi Anbouhi M., Hassanzadeh-Ghassabeh G., Muyldermans S. Generation and characterization of a functional nanobody against the vascular endothelial growth factor receptor-2; angiogenesis cell receptor. Mol. Immunol. 2012;50:35–41. doi: 10.1016/j.molimm.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Behdani M., Zeinali S., Karimipour M., Khanahmad H., Schoonooghe S., Aslemarz A., Seyed N., Moazami-Godarzi R., Baniahmad F., Habibi-Anbouhi M., Hassanzadeh-Ghassabeh G., Muyldermans S. Development of VEGFR2-specific Nanobody Pseudomonas exotoxin A conjugated to provide efficient inhibition of tumor cell growth. N. Biotechnol. 2013;30:205–209. doi: 10.1016/j.nbt.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Blanchetot C., Verzijl D., Mujic-Delic A., Bosch L., Rem L., Leurs R., Verrips C.T., Saunders M., De Haard H., Smit M.J. Neutralizing nanobodies targeting diverse chemokines effectively inhibit chemokine function. J. Biol. Chem. 2013;288:25173–25182. doi: 10.1074/jbc.M113.467969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty R., Goel S., Cai W. Nanobody: the “magic bullet” for molecular imaging? Theranostics. 2014;4:386–398. doi: 10.7150/thno.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatalic K.L., Veldhoven-Zweistra J., Bolkestein M., Hoeben S., Koning G.A., Boerman O.C., De Jong M., Van Weerden W.M. A novel (1)(1)(1)In-labeled anti-prostate-specific membrane antigen nanobody for targeted SPECT/CT imaging of prostate cancer. J. Nucl. Med. 2015;56:1094–1099. doi: 10.2967/jnumed.115.156729. [DOI] [PubMed] [Google Scholar]
- Chen J., He Q.H., Xu Y., Fu J.H., Li Y.P., Tu Z., Wang D., Shu M., Qiu Y.L., Yang H.W., Liu Y.Y. Nanobody medicated immunoassay for ultrasensitive detection of cancer biomarker alpha-fetoprotein. Talanta. 2016;147:523–530. doi: 10.1016/j.talanta.2015.10.027. [DOI] [PubMed] [Google Scholar]
- De Buck S., Nolf J., De Meyer T., Virdi V., De Wilde K., Van Lerberge E., Van Droogenbroeck B., Depicker A. Fusion of an Fc chain to a VHH boosts the accumulation levels in Arabidopsis seeds. Plant Biotechnol. J. 2013;11:1006–1016. doi: 10.1111/pbi.12094. [DOI] [PubMed] [Google Scholar]
- De Meyer T., Muyldermans S., Depicker A. Nanobody-based products as research and diagnostic tools. Trends Biotechnol. 2014;32:263–270. doi: 10.1016/j.tibtech.2014.03.001. [DOI] [PubMed] [Google Scholar]
- D'Huyvetter M., Aerts A., Xavier C., Vaneycken I., Devoogdt N., Gijs M., Impens N., Baatout S., Ponsard B., Muyldermans S., Caveliers V., Lahoutte T. Development of 177Lu-nanobodies for radioimmunotherapy of HER2-positive breast cancer: evaluation of different bifunctional chelators. Contrast Media Mol. Imaging. 2012;7:254–264. doi: 10.1002/cmmi.491. [DOI] [PubMed] [Google Scholar]
- D'Huyvetter M., Vincke C., Xavier C., Aerts A., Impens N., Baatout S., De Raeve H., Muyldermans S., Caveliers V., Devoogdt N., Lahoutte T. Targeted radionuclide therapy with a177Lu-labeled anti-HER2 nanobody. Theranostics. 2014;4:708–720. doi: 10.7150/thno.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimizadeh W., Mousavi Gargari S.L., Javidan Z., Rajabibazl M. Production of novel VHH nanobody inhibiting angiogenesis by targeting binding site of VEGF. Appl. Biochem. Biotechnol. 2015;176:1985–1995. doi: 10.1007/s12010-015-1695-y. [DOI] [PubMed] [Google Scholar]
- Evazalipour M., D'Huyvetter M., Tehrani B.S., Abolhassani M., Omidfar K., Abdoli S., Arezumand R., Morovvati H., Lahoutte T., Muyldermans S., Devoogdt N. Generation and characterization of nanobodies targeting PSMA for molecular imaging of prostate cancer. Contrast Media Mol. Imaging. 2014;9:211–220. doi: 10.1002/cmmi.1558. [DOI] [PubMed] [Google Scholar]
- Even-Desrumeaux K., Fourquet P., Secq V., Baty D., Chames P. Single-domain antibodies: a versatile and rich source of binders for breast cancer diagnostic approaches. Mol. Biosyst. 2012;8:2385–2394. doi: 10.1039/c2mb25063b. [DOI] [PubMed] [Google Scholar]
- Fan X., Wang L., Guo Y., Tu Z., Li L., Tong H., Xu Y., Li R., Fang K. Ultrasonic nanobubbles carrying anti-PSMA nanobody: construction and application in prostate cancer-targeted imaging. PLoS One. 2015;10:e0127419. doi: 10.1371/journal.pone.0127419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajpour Z., Rahbarizadeh F., Kazemi B., Ahmadvand D. A nanobody directed to a functional epitope on VEGF, as a novel strategy for cancer treatment. Biochem. Biophys. Res. Commun. 2014;446:132–136. doi: 10.1016/j.bbrc.2014.02.069. [DOI] [PubMed] [Google Scholar]
- Hamers-Casterman C., Atarhouch T., Muyldermans S., Robinson G., Hamers C., Songa E.B., Bendahman N., Hamers R. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- Herce H.D., Deng W., Helma J., Leonhardt H., Cardoso M.C. Visualization and targeted disruption of protein interactions in living cells. Nat. Commun. 2013;4:2660. doi: 10.1038/ncomms3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernot S., Unnikrishnan S., Du Z., Cosyns B., Broisat A., Muyldermans S., Lahoutte T., Klibanov A.L., Devoogdt N. Nanobody-coupled microbubbles as novel molecular tracer. Eur. Heart J. 2012;33:403–404. doi: 10.1016/j.jconrel.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heukers R., Altintas I., Raghoenath S., De Zan E., Pepermans R., Roovers R.C., Haselberg R., Hennink W.E., Schiffelers R.M., Kok R.J., Van Bergen En Henegouwen P.M. Targeting hepatocyte growth factor receptor (Met) positive tumor cells using internalizing nanobody-decorated albumin nanoparticles. Biomaterials. 2014;35:601–610. doi: 10.1016/j.biomaterials.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Heukers R., Van Bergen En Henegouwen P.M., Oliveira S. Nanobody-photosensitizer conjugates for targeted photodynamic therapy. Nanomedicine. 2014;10:1441–1451. doi: 10.1016/j.nano.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Huet H.A., Growney J.D., Johnson J.A.0., Li J., Bilic S., Ostrom L., Zafari M., Kowal C., Yang G., Royo A., Jensen M., Dombrecht B., Meerschaert K.R., Kolkman J.A., Cromie K.D., Mosher R., Gao H., Schuller A., Isaacs R., Sellers W.R., Ettenberg S.A. Multivalent nanobodies targeting death receptor 5 elicit superior tumor cell killing through efficient caspase induction. MAbs. 2014;6:1560–1570. doi: 10.4161/19420862.2014.975099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnichen S., Blanchetot C., Maussang D., Gonzalez-Pajuelo M., Chow K.Y., Bosch L., De Vrieze S., Serruys B., Ulrichts H., Vandevelde W., Saunders M., De Haard H.J., Schols D., Leurs R., Vanlandschoot P., Verrips T., Smit M.J. CXCR4 nanobodies (VHH-based single variable domains) potently inhibit chemotaxis and HIV-1 replication and mobilize stem cells. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20565–20570. doi: 10.1073/pnas.1012865107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamnani F.R., Rahbarizadeh F., Shokrgozar M.A., Ahmadvand D., Mahboudi F., Sharifzadeh Z. Targeting high affinity and epitope-distinct oligoclonal nanobodies to HER2 over-expressing tumor cells. Exp. Cell Res. 2012;318:1112–1124. doi: 10.1016/j.yexcr.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Jovcevska I., Zupanec N., Kocevar N., Cesselli D., Podergajs N., Stokin C.L., Myers M.P., Muyldermans S., Ghassabeh G.H., Motaln H., Ruaro M.E., Bourkoula E., Turnsek T.L., Komel R. TRIM28 and beta-actin identified via nanobody-based reverse proteomics approach as possible human glioblastoma biomarkers. PLoS One. 2014;9:e113688. doi: 10.1371/journal.pone.0113688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek J.Z., Skottrup P.D. Selection and characterization of camelid nanobodies towards urokinase-type plasminogen activator. Mol. Immunol. 2015;65:384–390. doi: 10.1016/j.molimm.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Kazemi-Lomedasht F., Behdani M., Bagheri K.P., Habibi-Anbouhi M., Abolhassani M., Arezumand R., Shahbazzadeh D., Mirzahoseini H. Inhibition of angiogenesis in human endothelial cell using VEGF specific nanobody. Mol. Immunol. 2015;65:58–67. doi: 10.1016/j.molimm.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Keyaerts M., Xavier C., Heemskerk J., Devoogdt N., Everaert H., Ackaert C., Vanhoeij M., Duhoux F.P., Gevaert T., Simon P., Schallier D., Fontaine C., Vaneycken I., Vanhove C., De Greve J., Lamote J., Caveliers V., Lahoutte T. Phase I study of 68Ga-HER2-nanobody for PET/CT assessment of HER2-expression in breast carcinoma. J. Nucl. Med. 2015 doi: 10.2967/jnumed.115.162024. [DOI] [PubMed] [Google Scholar]
- Kijanka M., Warnders F.J., El Khattabi M., Lub-De Hooge M., Van Dam G.M., Ntziachristos V., De Vries L., Oliveira S., Henegouwen P.M.P.V.E. Rapid optical imaging of human breast tumour xenografts using anti-HER2 VHHs site-directly conjugated to IRDye 800CW for image-guided surgery. Eur. J. Nucl. Med. Mol. Imaging. 2013;40:1718–1729. doi: 10.1007/s00259-013-2471-2. [DOI] [PubMed] [Google Scholar]
- Kijanka M., Dorresteijn B., Oliveira S., Van Bergen En Henegouwen P.M. Nanobody-based cancer therapy of solid tumors. Nanomedicine (Lond.) 2015;10:161–174. doi: 10.2217/nnm.14.178. [DOI] [PubMed] [Google Scholar]
- Li T., Bourgeois J.P., Celli S., Glacial F., Le Sourd A.M., Mecheri S., Weksler B., Romero I., Couraud P.O., Rougeon F., Lafaye P. Cell-penetrating anti-GFAP VHH and corresponding fluorescent fusion protein VHH-GFP spontaneously cross the blood-brain barrier and specifically recognize astrocytes: application to brain imaging. FASEB J. 2012;26:3969–3979. doi: 10.1096/fj.11-201384. [DOI] [PubMed] [Google Scholar]
- Maier J., Traenkle B., Rothbauer U. Real-time analysis of epithelial-mesenchymal transition using fluorescent single-domain antibodies. Sci. Rep. 2015;5:13402. doi: 10.1038/srep13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa S., Xavier C., De Vos J., Caveliers V., Lahoutte T., Muyldermans S., Devoogdt N. Site-specific labeling of cysteine-tagged camelid single-domain antibody-fragments for use in molecular imaging. Bioconjug. Chem. 2014;25:979–988. doi: 10.1021/bc500111t. [DOI] [PubMed] [Google Scholar]
- Maussang, D., Mujic-Delic, A., Descamps, F. J., Stortelers, C., Vanlandschoot P., Stigter-Van Walsum M., Vischer, H. F., Van Roy, M., Vosjan, M., Gonzalez-Pajuelo, M., Van Dongen, G. A., Merchiers, P., Van Rompaey, P. & Smit, M. J. 2013. Llama-derived single variable domains (nanobodies) directed against chemokine receptor CXCR7 reduce head and neck cancer cell growth in vivo. J. Biol. Chem., 288, 29562–72. [DOI] [PMC free article] [PubMed]
- Mikhaylova M., Cloin B.M., Finan K., Van Den Berg R., Teeuw J., Kijanka M.M., Sokolowski M., Katrukha E.A., Maidorn M., Opazo F., Moutel S., Vantard M., Perez F., Van Bergen En Henegouwen P.M., Hoogenraad C.C., Ewers H., Kapitein L.C. Resolving bundled microtubules using anti-tubulin nanobodies. Nat. Commun. 2015;6:7933. doi: 10.1038/ncomms8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahedi K., Schoonooghe S., Laoui D., Houbracken I., Waelput W., Breckpot K., Bouwens L., Lahoutte T., De Baetselier P., Raes G., Devoogdt N., Van Ginderachter J.A. Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Res. 2012;72:4165–4177. doi: 10.1158/0008-5472.CAN-11-2994. [DOI] [PubMed] [Google Scholar]
- Muyldermans S. Nanobodies: natural single-domain antibodies. Annu. Rev. Biochem. 2013;82:775–797. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- Newnham L.E., Wright M.J., Holdsworth G., Kostarelos K., Robinson M.K., Rabbitts T.H., Lawson A.D. Functional inhibition of beta-catenin-mediated Wnt signaling by intracellular VHH antibodies. MAbs. 2015;7:180–191. doi: 10.4161/19420862.2015.989023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira S., Schiffelers R.M., van der Veeken J., van der Meel R., Vongpromek R., Van Bergen En Henegouwen P.M., Storm G., Roovers R.C. Downregulation of EGFR by a novel multivalent nanobody-liposome platform. J. Control. Release. 2010;145:165–175. doi: 10.1016/j.jconrel.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Oliveira S., Heukers R., Sornkom J., Kok R.J., Van Bergen En Henegouwen P.M. Targeting tumors with nanobodies for cancer imaging and therapy. J. Control. Release. 2013;172:607–617. doi: 10.1016/j.jconrel.2013.08.298. [DOI] [PubMed] [Google Scholar]
- Omidfar K., Amjad Zanjani F.S., Hagh A.G., Azizi M.D., Rasouli S.J., Kashanian S. Efficient growth inhibition of EGFR over-expressing tumor cells by an anti-EGFR nanobody. Mol. Biol. Rep. 2013;40:6737–6745. doi: 10.1007/s11033-013-2790-1. [DOI] [PubMed] [Google Scholar]
- Papadopoulos K.P., Isaacs R., Bilic S., Kentsch K., Huet H.A., Hofmann M., Rasco D., Kundamal N., Tang Z., Cooksey J., Mahipal A. Unexpected hepatotoxicity in a phase I study of TAS266, a novel tetravalent agonistic nanobody(R) targeting the DR5 receptor. Cancer Chemother. Pharmacol. 2015;75:887–895. doi: 10.1007/s00280-015-2712-0. [DOI] [PubMed] [Google Scholar]
- Patris S., De Pauw P., Vandeput M., Huet J., Van Antwerpen P., Muyldermans S., Kauffmann J.M. Nanoimmunoassay onto a screen printed electrode for HER2 breast cancer biomarker determination. Talanta. 2014;130:164–170. doi: 10.1016/j.talanta.2014.06.069. [DOI] [PubMed] [Google Scholar]
- Pruszynski M., Koumarianou E., Vaidyanathan G., Revets H., Devoogdt N., Lahoutte T., Zalutsky M.R. Targeting breast carcinoma with radioiodinated anti-HER2 nanobody. Nucl. Med. Biol. 2013;40:52–59. doi: 10.1016/j.nucmedbio.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski M., Koumarianou E., Vaidyanathan G., Revets H., Devoogdt N., Lahoutte T., Lyerly H.K., Zalutsky M.R. Improved tumor targeting of anti-HER2 nanobody through N-succinimidyl 4-guanidinomethyl-3-iodobenzoate radiolabeling. J. Nucl. Med. 2014;55:650–656. doi: 10.2967/jnumed.113.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roovers R.C., Laeremans T., Huang L., De Taeye S., Verkleij A.J., Revets H., De Haard H.J., Van Bergen En Henegouwen P.M. Efficient inhibition of EGFR signaling and of tumour growth by antagonistic anti-EFGR nanobodies. Cancer Immunol. Immunother. 2007;56:303–317. doi: 10.1007/s00262-006-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roovers R.C., Vosjan M.J., Laeremans T., El Khoulati R., De Bruin R.C., Ferguson K.M., Verkleij A.J., Van Dongen G.A., Van Bergen En Henegouwen P.M. A biparatopic anti-EGFR nanobody efficiently inhibits solid tumour growth. Int. J. Cancer. 2011;129:2013–2024. doi: 10.1002/ijc.26145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruano-Gallego D., Alvarez B., Fernandez L.A. Engineering the controlled assembly of filamentous injectisomes in E. coli K-12 for protein translocation into mammalian cells. ACS Synth. Biol. 2015;4:1030–1041. doi: 10.1021/acssynbio.5b00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeqzadeh E., Rahbarizadeh F., Ahmadvand D., Rasaee M.J., Parhamifar L., Moghimi S.M. Combined MUC1-specific nanobody-tagged PEG-polyethylenimine polyplex targeting and transcriptional targeting of tBid transgene for directed killing of MUC1 over-expressing tumour cells. J. Control. Release. 2011;156:85–91. doi: 10.1016/j.jconrel.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Schmitz K.R., Bagchi A., Roovers R.C., Van Bergen En Henegouwen P.M., Ferguson K.M. Structural evaluation of EGFR inhibition mechanisms for nanobodies/VHH domains. Structure. 2013;21:1214–1224. doi: 10.1016/j.str.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifzadeh Z., Rahbarizadeh F., Shokrgozar M.A., Ahmadvand D., Mahboudi F., Rahimi Jamnani F., Aghaee Bakhtiari S.H. Development of oligoclonal nanobodies for targeting the tumor-associated glycoprotein 72 antigen. Mol. Biotechnol. 2013;54:590–601. doi: 10.1007/s12033-012-9601-0. [DOI] [PubMed] [Google Scholar]
- Slordahl T.S., Denayer T., Moen S.H., Standal T., Borset M., Ververken C., Ro T.B. Anti-c-MET nanobody - a new potential drug in multiple myeloma treatment. Eur. J. Haematol. 2013;91:399–410. doi: 10.1111/ejh.12185. [DOI] [PubMed] [Google Scholar]
- Summanen M., Granqvist N., Tuominen R.K., Yliperttula M., Verrips C.T., Boonstra J., Blanchetot C., Ekokoski E. Kinetics of PKCepsilon activating and inhibiting llama single chain antibodies and their effect on PKCepsilon translocation in HeLa cells. PLoS One. 2012;7:e35630. doi: 10.1371/journal.pone.0035630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli M., Rijcken C.J., Oliveira S., van der Meel R., Van Bergen En Henegouwen P.M., Lammers T., Van Nostrum C.F., Storm G., Hennink W.E. Nanobody-shell functionalized thermosensitive core-crosslinked polymeric micelles for active drug targeting. J. Control. Release. 2011;151:183–192. doi: 10.1016/j.jconrel.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Talelli M., Oliveira S., Rijcken C.J., Pieters E.H., Etrych T., Ulbrich K., Van Nostrum R.C., Storm G., Hennink W.E., Lammers T. Intrinsically active nanobody-modified polymeric micelles for tumor-targeted combination therapy. Biomaterials. 2013;34:1255–1260. doi: 10.1016/j.biomaterials.2012.09.064. [DOI] [PubMed] [Google Scholar]
- Tijink B.M., Laeremans T., Budde M., Stigter Van Walsum M., Dreier T., De Haard H.J., Leemans C.R., Van Dongen G.A. Improved tumor targeting of anti-epidermal growth factor receptor nanobodies through albumin binding: taking advantage of modular nanobody technology. Mol. Cancer Ther. 2008;7:2288–2297. doi: 10.1158/1535-7163.MCT-07-2384. [DOI] [PubMed] [Google Scholar]
- van Audenhove I., Boucherie C., Pieters L., Zwaenepoel O., Vanloo B., Martens E., Verbrugge C., Hassanzadeh-Ghassabeh G., Vandekerckhove J., Cornelissen M., De Ganck A., Gettemans J. Stratifying fascin and cortactin function in invadopodium formation using inhibitory nanobodies and targeted subcellular delocalization. FASEB J. 2014;28:1805–1818. doi: 10.1096/fj.13-242537. [DOI] [PubMed] [Google Scholar]
- van Brussel A.S., Adams A., Oliveira S., Dorresteijn B., El Khattabi M., Vermeulen J.F., Van Der Wall E., Mali W.P., Derksen P.W., Van Diest P.J., Van Bergen En Henegouwen P.M. Hypoxia-targeting fluorescent nanobodies for optical molecular imaging of pre-invasive breast cancer. Mol. Imaging Biol. 2015 doi: 10.1007/s11307-015-0909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meel R., Oliveira S., Altintas I., Haselberg R., Van Der Veeken J., Roovers R.C., Van Bergen En Henegouwen P.M., Storm G., Hennink W.E., Schiffelers R.M., Kok R.J. Tumor-targeted nanobullets: anti-EGFR nanobody-liposomes loaded with anti-IGF-1R kinase inhibitor for cancer treatment. J. Control. Release. 2012;159:281–289. doi: 10.1016/j.jconrel.2011.12.027. [DOI] [PubMed] [Google Scholar]
- van der Meel R., Oliveira S., Altintas I., Heukers R., Pieters E.H., Van Bergen En Henegouwen P.M., Storm G., Hennink W.E., Kok R.J., Schiffelers R.M. Inhibition of tumor growth by targeted anti-EGFR/IGF-1R nanobullets depends on efficient blocking of cell survival pathways. Mol. Pharm. 2013;10:3717–3727. doi: 10.1021/mp400212v. [DOI] [PubMed] [Google Scholar]
- van Driel P.B., van der Vorst J.R., Verbeek F.P., Oliveira S., Snoeks T.J., Keereweer S., Chan B., Boonstra M.C., Frangioni J.V., Van Bergen En Henegouwen P.M., Vahrmeijer A.L., Lowik C.W. Intraoperative fluorescence delineation of head and neck cancer with a fluorescent anti-epidermal growth factor receptor nanobody. Int. J. Cancer. 2014;134:2663–2673. doi: 10.1002/ijc.28601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Impe K., Bethuyne J., Cool S., Impens F., Ruano-Gallego D., De Wever O., Vanloo B., Van Troys M., Lambein K., Boucherie C., Martens E., Zwaenepoel O., Hassanzadeh-Ghassabeh G., Vandekerckhove J., Gevaert K., Fernandez L.A., Sanders N.N., Gettemans J. A nanobody targeting the F-actin capping protein CapG restrains breast cancer metastasis. Breast Cancer Res. 2013;15:R116. doi: 10.1186/bcr3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosjan M.J.W.D., Perk L.R., Roovers R.C., Visser G.W.M., Stigter Van Walsum M., Henegouwen P.M.P.V.E., Van Dongen G.A.M.S. Facile labelling of an anti-epidermal growth factor receptor nanobody with Ga-68 via a novel bifunctional desferal chelate for immuno-PET. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:753–763. doi: 10.1007/s00259-010-1700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosjan M.J., Vercammen J., Kolkman J.A., Stigter Van Walsum M., Revets H., Van Dongen G.A. Nanobodies targeting the hepatocyte growth factor: potential new drugs for molecular cancer therapy. Mol. Cancer Ther. 2012;11:1017–1025. doi: 10.1158/1535-7163.MCT-11-0891. [DOI] [PubMed] [Google Scholar]
- Xavier C., Vaneycken I., D'Huyvetter M., Heemskerk J., Keyaerts M., Vincke C., Devoogdt N., Muyldermans S., Lahoutte T., Caveliers V. Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 nanobodies for iPET imaging of HER2 receptor expression in cancer. J. Nucl. Med. 2013;54:776–784. doi: 10.2967/jnumed.112.111021. [DOI] [PubMed] [Google Scholar]
- Zare H., Rajabibazl M., Rasooli I., Ebrahimizadeh W., Bakherad H., Ardakani L.S., Gargari S.L. Production of nanobodies against prostate-specific membrane antigen (PSMA) recognizing LnCaP cells. Int. J. Biol. Markers. 2014;29:e169–e179. doi: 10.5301/jbm.5000063. [DOI] [PubMed] [Google Scholar]
- Zou T., Dembele F., Beugnet A., Sengmanivong L., Trepout S., Marco S., Marco A., Li M.H. Nanobody-functionalized PEG-b-PCL polymersomes and their targeting study. J. Biotechnol. 2015;214:147–155. doi: 10.1016/j.jbiotec.2015.09.034. [DOI] [PubMed] [Google Scholar]