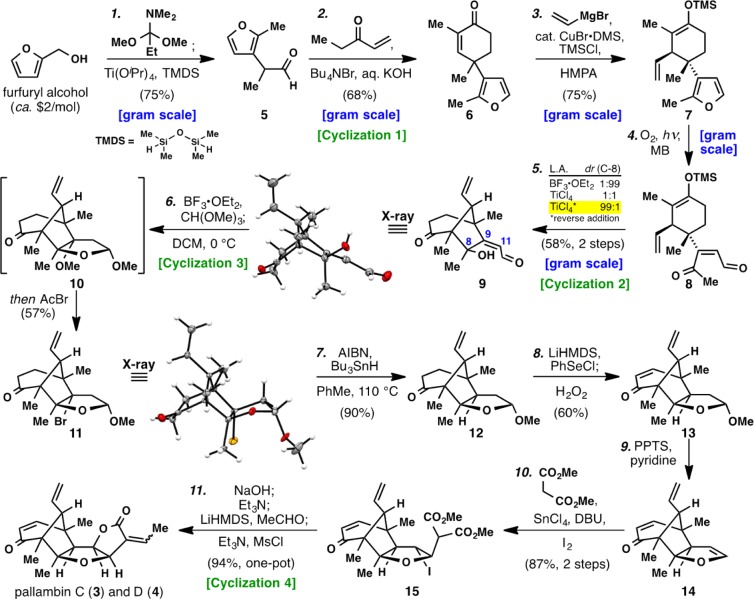
Scheme 1. Total Synthesis of Pallambins C (3) and D (4).
Reagents and conditions: (1) furfuryl alcohol (1 equiv), 1,1-dimethoxy-N,N-dimethylpropan-1-amine (1.5 equiv), PhMe, 110 °C then TMDS (2 equiv), Ti(OiPr)4 (1.5 equiv), 50 °C (75%, one-pot); (2) ethyl vinyl ketone (1.5 equiv), Bu4NBr (10 mol %), 60% aq. KOH, PhMe, 23 °C (68%); (3) vinyl magnesium bromide (3.5 equiv), CuBr·DMS (20 mol %), HMPA (4 equiv), TMSCl (1.1 equiv), THF, −78 °C (75%); (4) O2, methylene blue, hν, DCM, −10 °C, then thiourea (1.5 equiv), 23 °C; (5) TiCl4 (1.5 equiv), Et2O, −78 °C (58%, 2 steps); (6) CH(OMe)3 (1.5 equiv), BF3·OEt2 (1.1 equiv), MgSO4 (25 equiv), DCM, 0 °C then AcBr (1 equiv) (57%); (7) Bu3SnH (1.5 equiv), AIBN (1 equiv), PhMe, 110 °C (90%); (8) LiHMDS (2 equiv), PhSeCl (2 equiv), THF, −78 °C then H2O2 (5.0 equiv), 0 °C (60%, one-pot); (9) PPTS (4 equiv), pyridine (4 equiv), PhCl, 130 °C; (10) dimethyl malonate (5 equiv), SnCl4 (5 equiv), DBU (5 equiv), I2 (1 equiv), DCM, 23 °C (87%, 2 steps); (11) 2 M NaOH, MeOH, 23 °C then Et3N (10.0 equiv), MeCN, 60 °C then LiHMDS (2.5 equiv), MeCHO (5 equiv), THF, −78 °C then Et3N (30 equiv), MsCl (5.0 equiv), DMAP, DCM, 23 °C (94%, one-pot); TMDS = 1,1,3,3-tetramethyldisiloxane, EVK = ethyl vinyl ketone, DMS = dimethyl sulfide, HMPA = hexamethylphosphoramide, MB = methylene blue, DCM = dichloromethane, AIBN = 2,2′-azobis(2-methylpropionitrile), DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene, LiHMDS = lithium bis(trimethylsilyl)amide, PPTS = pyridinium p-toluenesulfonate, DMAP = 4-(dimethylamino)pyridine