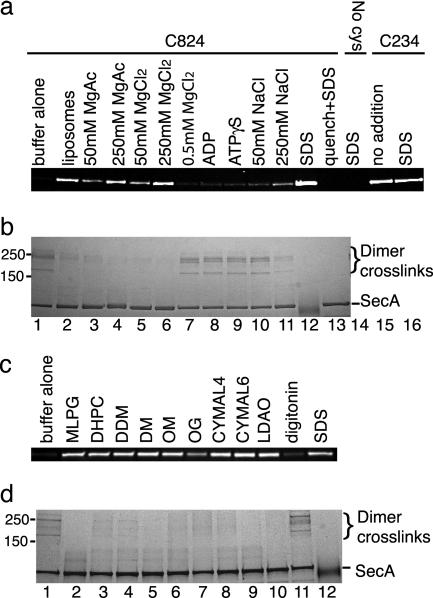
Fig. 5.
E. coli SecA can adopt an open conformation in solution. (a) SecA containing single cysteines at positions 824 or 234, or a mutant lacking cysteines (no cys), were labeled with maleimide fluorescein in the presence of the indicated additions. When nucleotide (0.25 mM) was added, 0.5 mM MgCl2 was also included. The samples were separated by SDS/PAGE and visualized under UV light. In the lane labeled quench+SDS, the quenching reagent, either DTT or glycine, was added before labeling or crosslinking. (b) In parallel, samples were crosslinked with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, separated by SDS/PAGE, and stained with Coomassie blue. The position of dimer crosslinks is indicated. (c) Modification reactions with SecA containing a cysteine at position 824 were performed as in a in the presence of 1-myristoyl-2-hydroxy-sn-glycero-3-[phospho-rac-(1-glycerol)] (MLPG, 0.1 mM); 1,2-diheptanoyl-sn-glycero-3-phosphocholine (DHPC, 4.2 mM); dodecyl maltoside (DDM, 0.6 mM); decyl maltoside (DM, 3.6 mM); octyl maltoside (OM, 39 mM); octyl glucoside (OG, 36.4 mM); CYMAL4 (15.2 mM); CYMAL6 (1.12 mM); lauryldimethylamine-N-oxide (LDAO, 2 mM); digitonin (1%); or SDS (0.5%). (d) In parallel, samples were crosslinked with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide.