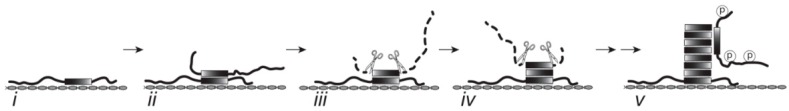
Figure 6.
Schematic representation of tau-tau binding. (i) Tau is initially captured by binding to a solid-phase substrate. The identity of the endogenous substrate in the aging brain is unknown, but may be formed from macromolecular complexes which escape normal endosomal/lysosomal processing with advancing age; (ii) The tau bound to a solid-phase substrate is able to capture further full-length tau through a tau-tau binding interaction which has greater affinity (lower energy) than the physiological tau-tubulin binding interaction; (iii) This locks the repeat domain into a proteolytically stable configuration such that proteolytic cleavage of the N- and C-termini leaves behind a characteristic tau fragment restricted to the repeat domain, the core tau unit of the PHF; (iv) The oligomeric form of truncated tau is able to propagate capture and proteolytic processing of further tau, and repeated cycles of binding and proteolysis result in accumulation of PHF-core tau; (v) It is only at a later stage that non-proteolysed, full-length tau molecules are added and these become secondarily phosphorylated. The optimal points of pharmaceutical intervention are therefore blocking the binding of tau to the initiating endogenous substrate or blocking the propagation of tau aggregation cascade with tau aggregation inhibitors.