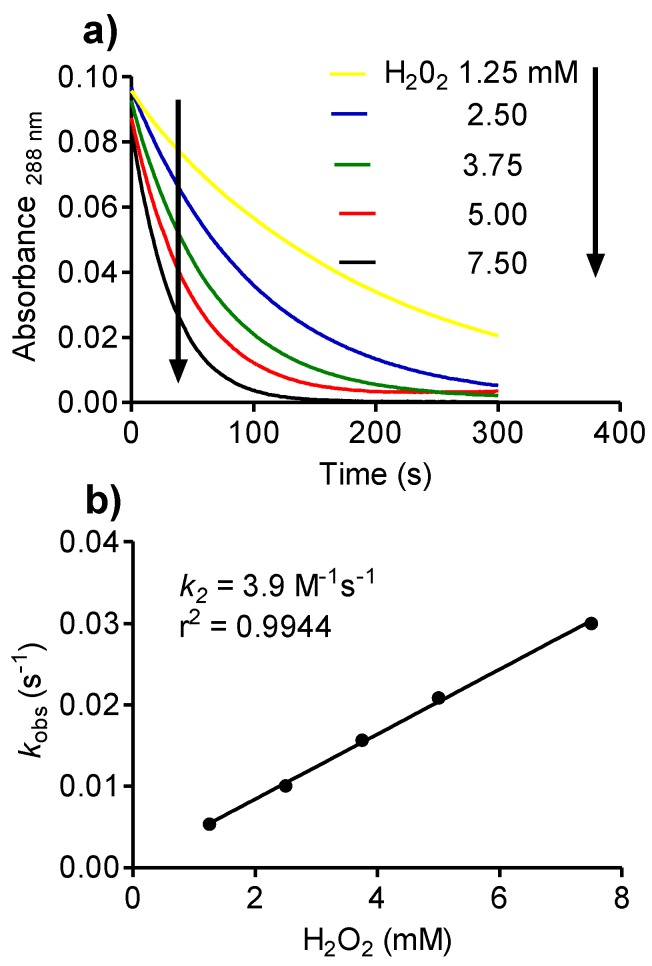
Figure 9.
Reactivity of Tau-NHBr with H2O2. (a) Kinetic profile of Tau-NHBr consumption for determination of kobs under pseudo-first-order experimental conditions. The reaction mixture was composed of 250 µM Tau-NHBr and increasing concentrations of H2O2 in 50 mM phosphate buffer, pH 7.0; (b) Determination of the bimolecular rate constant (k2) for the reaction between H2O2 and Tau-NHBr.