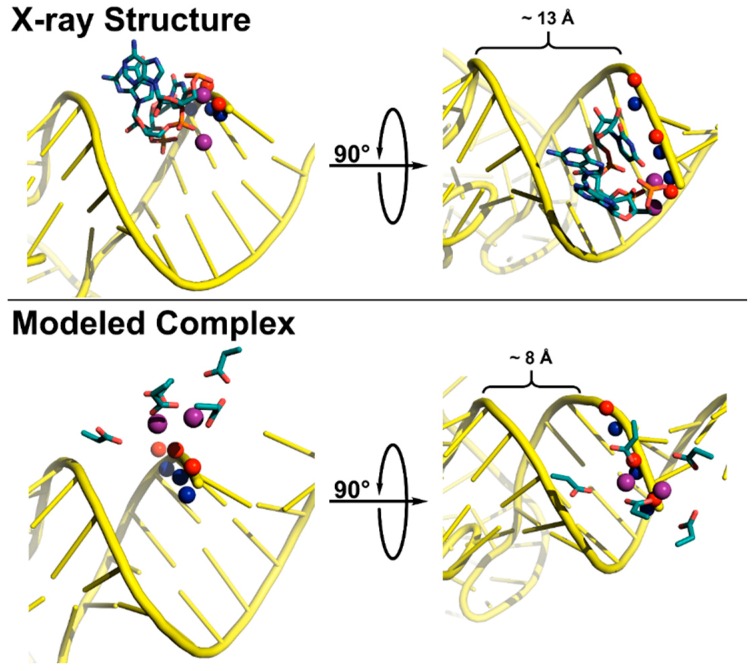
Figure 7.
Active site coordination of substrate by T. maritima RNase P (top, x-ray crystal structure) and A. thaliana PRORP1 (bottom, complex modeled in PyMOL). The minor groove width was measured as the distance between the non-bridging phosphate oxygens. Top: T. maritima product complex crystal structure (PDB 3q1r) [22]. Pro-RP (blue spheres) and pro-SP (red spheres) oxygen atoms of tRNA product (yellow cartoon) shown for N(+1)–N(+3). Active site metal atoms (purple spheres) and metal-coordinating residues A50, G51, and U52 are visualized (teal sticks). Bottom: The PRORP1 active site (PDB 4g24) was aligned to S. cerevisiae tRNAAsp (PDB 2tra) using the human DNA exonuclease I active site bound to DNA (PDB 3qeb) as a guide [64,153,154]. The tRNA, backbone oxygen atoms, active site metal atoms, and active site residues D399, D474/475, D493, and D497 are colored as in the top panel.