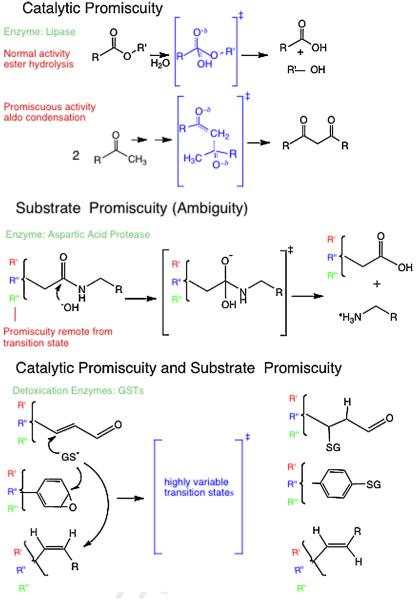
Fig. 2.
Chemical representation of promiscuous enzyme behaviors. Top: catalytic promiscuity includes structurally distinct transition states (in brackets) within the same active site. Structural differences also naturally occur remote from the transitions state for the different substrates (R vs. R′). Middle: substrate promiscuity refers to similar types of reactions, and hence similar local transition states (in brackets), for a single enzyme with a series of substrates that have structural differences remote from the local transition state (R, R′, R″). Bottom: bottom depicts the combination of catalytic and substrate promiscuity for detoxication enzymes that catalyze multiple types of reactions, with different local transition state structures (in brackets), and with variable structures remote from the transition state for any specific reaction type. For example the GSTs catalyze Michael type additions, addition to aryl epoxides (which subsequently aromatizes via dehydration), and cis-trans isomerization via addition–elimination, each for a wide range of substrate structures.