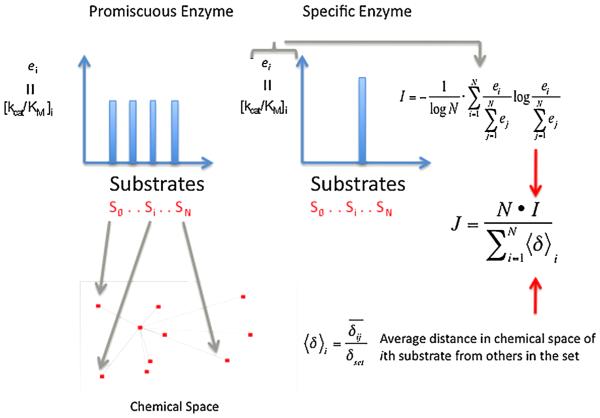
Fig. 3.
Schematized summary for calculating the promiscuity index, J. For a series of substrates the enzyme efficiency, ei = (kcat/KM) is determined and the term ‘I’ is calculated. I reflects the normalized probability that any given substrate will be chosen by an enzyme, when the enzyme is exposed simultaneously to low concentrations of each substrate, or the relative preference for any substrate. ‘I’ reflects the distribution of these preferences; a promiscuous enzyme with little preference (e1 = e2 = en) yields an I = 1 and a specific enzyme with a large e for only one substrate yields I = 0. It may be necessary to scale I to account for the chemical similarity or difference among the substrates used; if an enzyme has the same ei for all substrates that are far from one another in chemical space (chemically very different) than it is more promiscuous than an enzyme that has the same ei for substrates that sample a narrow range of chemical space (chemically similar). Substrates far away from others in chemical space, large <δ>i, add more weight to the calculated promiscuity, J. J = 1 for a perfectly promiscuous enzyme (no preference for any substrate) and J = 0 for a completely specific enzyme.