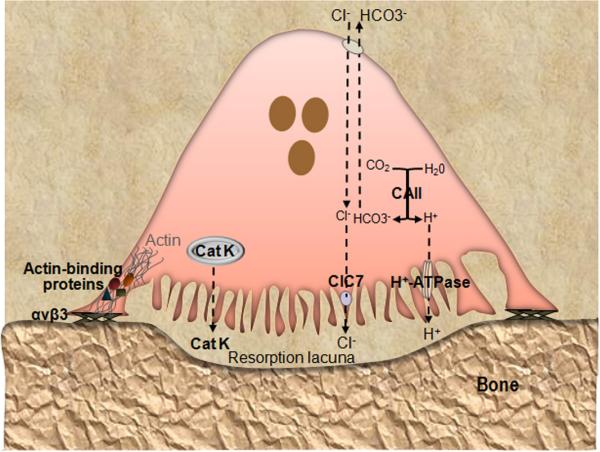
Figure 2.
Key molecules involved in OC function. Loss-of-function of any of the depicted molecules causes osteopetrosis due to defective OC activity. OC adhere to bone matrix proteins via integrin αvβ3 and polarized such that the plasma membrane facing bone is convoluted (ruffled) and contains the proton pump (v-ATPase) and Cl− channel 7 (ClC7), whereas the basolateral membrane is bears the HCO3−/Cl− antiporter. Cytoplasmic carbonic anhydrase type II (CAII) generates the protons to be secreted into the resorption lacuna beneath the cell. This lacuna becomes isolated from the rest of the extracellular space by the tight adhesion of αvβ3 to the bone surface at the sealing zone. The cytoplasmic domain of β3 recruits signaling proteins, which induce the association of actin with interacting partners (including talin, vinculin, kindlin, myosin IIA and paxillin), and formation of an actin ring that defines the periphery of the ruffled membrane. Concerted action of ClC7 and v-ATPase produces a high concentration of HCl that acidifies the resorption lacuna, leading to the dissolution of the inorganic components of the bone matrix. Acidified cytoplasmic vesicles containing lysosomal enzymes such as cathepsin K (Cat K) are also transported toward the bone-apposed plasma membrane, and ultimately, the sealed resorption lacuna, where they digest the exposed matrix proteins.