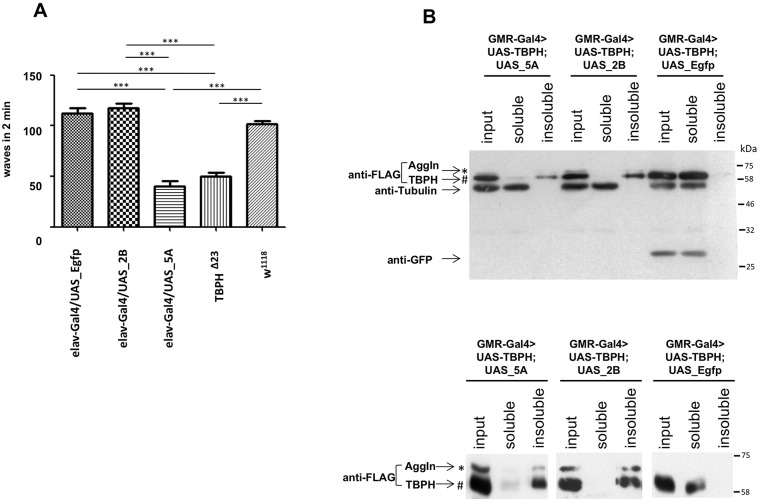
Fig. 6.
Effect of transgene expression on Drosophila larval motility and solubility assay on adult fly heads. (A) A larval motility assay was performed on third-instar larvae. A strong reduction in larval motility of elav-Gal4>UAS_5A (5A) larvae is observed, as compared to a transgenic line expressing the control protein EGFP (elav-Gal4>UAS_Egfp) and to the wild-type line (w1118). No impairment in larval motility is observed in elav-Gal4>UAS_2B larvae (2B). A TDP-43-null allele line (TBPHΔ23) was used as a positive reference control. x-axis, genotype; y-axis, peristaltic waves counted in two minutes. Error bars indicate s.e.m. (n=20 animals for each genotype). ***P<0.001 (one-way ANOVA). (B) Solubility assay. Western blot of fractionated proteins obtained from adult fly heads of the following genotypes: GMR-Gal4>UAS_TBPH; UAS_5A, GMR-Gal4>UAS_TBPH; UAS_2B, GMR-Gal4>UAS_TBPH; UAS_Egfp (TBPH is the Drosophila TDP-43). Upper panel, input, soluble and insoluble fractions of each genotype were loaded in a 1:1:1 ratio and probed by immunoblotting. AggIn and TBPH were detected with anti-FLAG antibody. EGFP was detected using anti-GFP antibody. Anti-tubulin served as protein loading control. Lower panel, to improve the separation of Flag-AggIn (see *) and Flag-TBPH (see #) protein bands, which have a close molecular mass, the three sample fractions from each genotype were also loaded on additional gels and were run for a longer time, before anti-Flag immunoblotting. TBPH is mostly insoluble when it is co-expressed with AggIn; by contrast, it remains mainly soluble when it is co-expressed with the unrelated protein EGFP.